
Metachromatic leukodystrophy (MLD) is a rare genetic disorder characterized by reduced arylsulfatase A (ARSA) enzyme activity, leading to the accumulation of sulfatides in the nervous system. This results in demyelination, neuroinflammation, and neurodegeneration, presenting as rapidly progressing clinical symptoms of neurodegenerative disease. Currently, there are no specific drugs available for MLD. Existing treatment strategies include ARSA enzyme replacement therapy, adeno-associated virus gene therapy, and allogeneic hematopoietic stem cell transplantation, but their efficacy is limited. In April 2024, Orchard Therapeutics' hematopoietic stem cell gene therapy product, Lenmeldy (Atidarsagene Autotemcel), was approved by the FDA. However, this therapy is only suitable for late infantile, pre-symptomatic, or early symptomatic adolescent MLD patients, limiting its benefit to a very small patient population. Due to a lack of awareness about MLD, most patients are diagnosed at a symptomatic stage. Unlike rapidly deteriorating late infantile MLD, later-onset types such as adolescent and adult MLD typically progress more slowly, offering a potential therapeutic window for intervention. Therefore, there is an urgent need to evaluate the safety and efficacy of HSCGT in treating symptomatic patients after symptom onset.
On June 25, 2024, a team led by Professor Lian Qizhou from Shenzhen University of Advanced Technology/Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, in collaboration with Guangzhou Women and Children's Medical Center, Shenzhen Second People's Hospital, Shenzhen Children's Hospital, and The University of Hong Kong, published their latest research findings in the journal Protein & Cell. The study, titled "Lentivirus-modified hematopoietic stem cell gene therapy for advanced symptomatic adolescent MLD: A long-term follow-up pilot study," reports nearly a decade-long safety and efficacy follow-up study of autologous hematopoietic stem cell gene therapy in symptomatic adolescent MLD patients.

Figure 1 Screenshot of the article online
Link:https://academic.oup.com/proteincell/advance-article/doi/10.1093/procel/pwae037/7698288?login=false
As early as 2014, Professor Lian Qizhou's team pioneered the first hematopoietic stem cell gene therapy in Asia for symptomatic adolescent MLD patients. They conducted a multicenter, single-arm, open-label clinical trial (ClinicalTrials.gov ID: NCT02559830) to evaluate the long-term safety of the therapy by analyzing adverse events occurring during short-term and long-term follow-ups. Clinical benefits were assessed through ARSA activity testing, MRI scoring, and neurological function scoring.
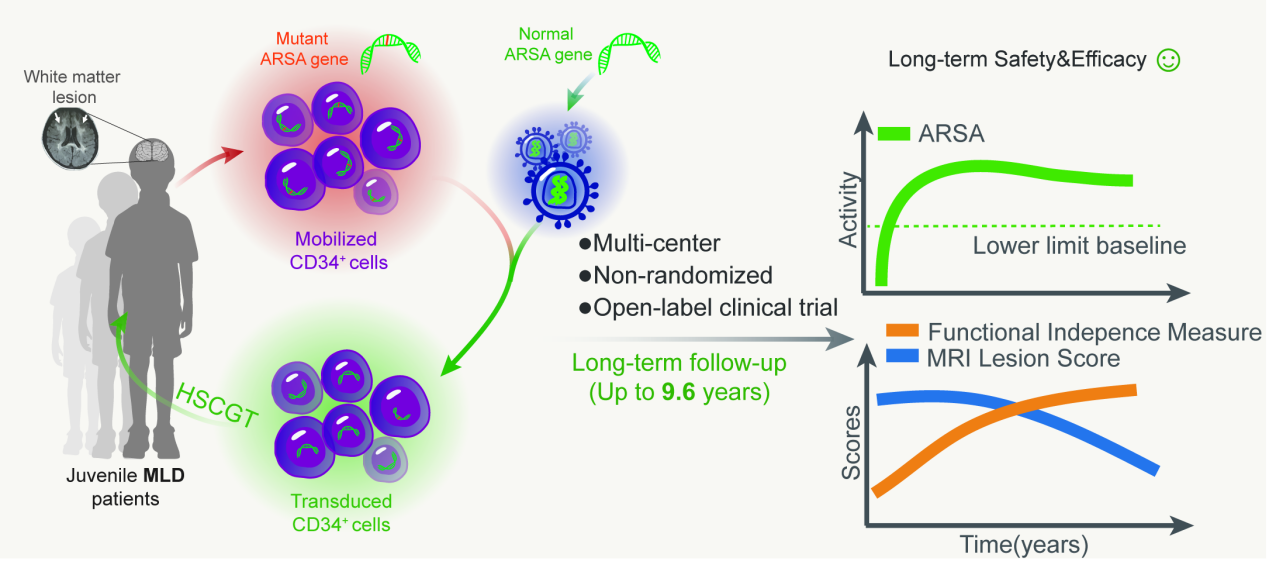
Figure 2 Conceptual Diagram of Lentiviral-Modified Hematopoietic Stem Cell Gene Therapy for Adolescent Patients after MLD Onset
This study screened six patients and is continuing to enroll participants. The research paper reported the clinical characteristics of the first three enrolled patients, along with safety and efficacy data followed up for 4.5, 6.9, and 9.6 years, respectively. Safety data analysis showed that adverse events occurring within two months after treatment were primarily neutropenia, related to chemotherapy myeloablation, which improved with conventional treatment in the short term. No HSCGT-related adverse events were observed during long-term follow-up, indicating good safety. Genomic integration efficiency assessment showed that functional ARSA genes were integrated into both hematopoietic stem cells and peripheral blood mononuclear cells of the patients. The research team paid special attention to mutations induced by random insertions of lentiviral vectors and the risk of oncogene activation. Through whole genome analysis and PCR detection, all 890 insertion sites were distributed across chromosomes without a tendency for clustered insertions, and most insertion sites were located within gene intron sequences. Only 12 insertion sites were found in exons, promoters, and 3’UTR regions, with no insertion sites detected in oncogenes.
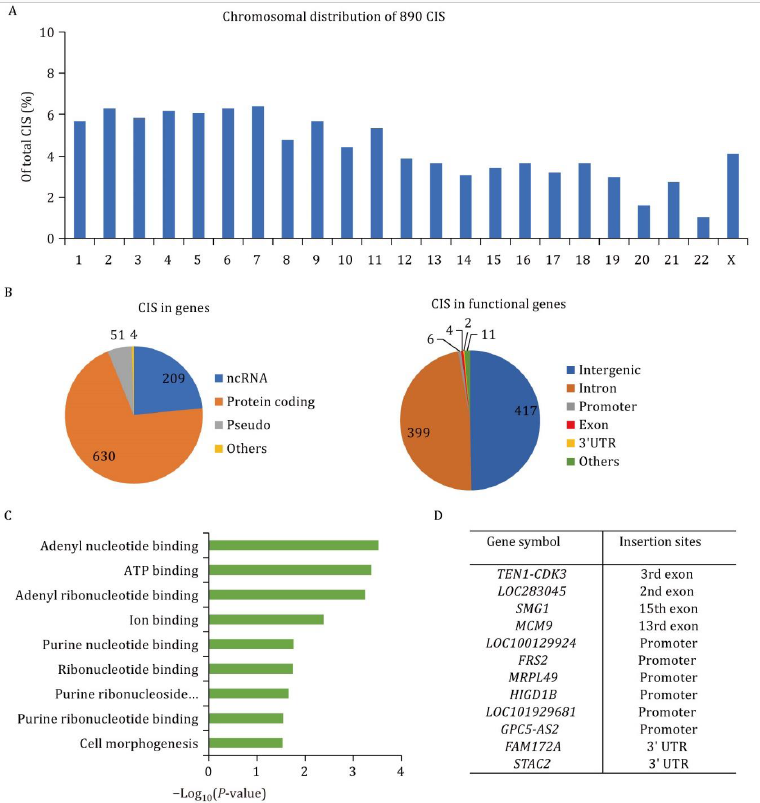
Figure 3. Whole-genome lentiviral insertion site analysis in MLD01 patient after gene therapy
Effectiveness evaluation showed that the serum ARSA enzyme activity of patients significantly increased after HSCGT and remained above normal levels. Compared to the natural course of the disease in untreated patients, the study patients showed improved cranial MRI scores after receiving HSCGT, indicating a stabilization of neurodegenerative disease progression. Neurological function scores, GMFC-MLD and FIM scores, improved after treatment, suggesting that HSCGT can enhance patients' daily living skills. The data from this study indicate that the HSCGT therapy developed by the research team can increase ARSA enzyme activity in symptomatic adolescent MLD patients, slow the progression of neurodegenerative diseases, improve patients' daily living skills, and has good safety. This study represents an important milestone in the field of gene therapy for genetic metabolic brain diseases and significantly advances the clinical translation of cell and gene therapies in China.
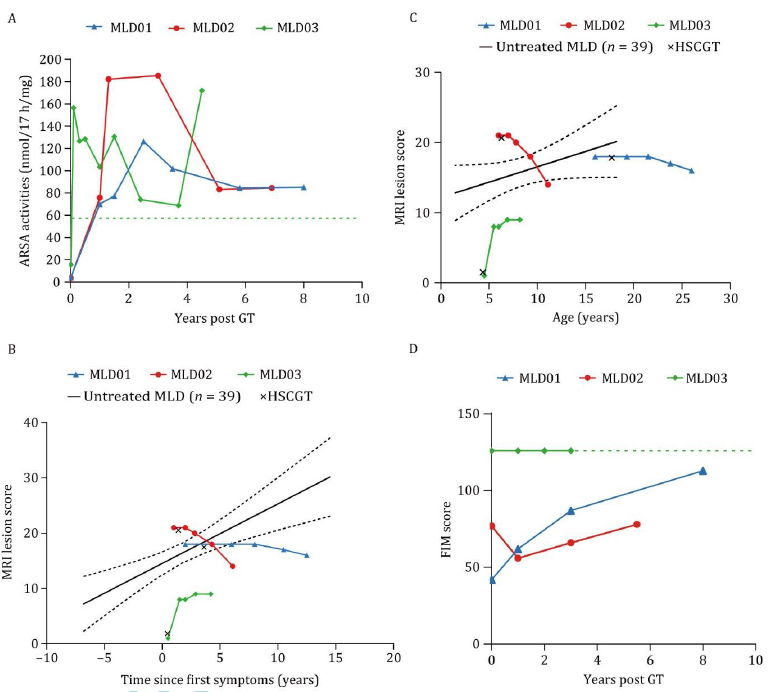
Figure 4. ARSA enzyme activity, MRI damage scores, and FIM scores before and after treatment in MLD patients
Professor Lian Qizhou from the Institute of Synthetic Biology at Shenzhen University of Advanced Technology/Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, was the corresponding author. Associate Researcher Zhang Zhao from Guangzhou Women's and Children's Medical Center/The University of Hong Kong (currently employed at Sun Yat-sen University), Professor Jiang Hua, co-trained PhD candidate Zhou Xiaoya from the SIAT and the University of Macau, Professor Liu Sixi from Shenzhen Children's Hospital were co-first authors. Professor Liu Hongsheng from Guangzhou Women's and Children's Medical Center, Professor Zhuojiacai, Professor Du Xin, and Professor Li Ming from Shenzhen Second People’s Hospital provided significant support for this study. This nearly decade-long study shows that lentiviral-modified hematopoietic stem cell gene therapy (HSCGT) is safe and effective for symptomatic adolescent MLD patients, offering new hope for effective treatment of the primary patient population.
Research Group Recruitment
Professor Lian Qizhou's research group focuses on:
1) Studying the molecular mechanisms of cardiovascular, vascular, and metabolic diseases using pluripotent stem cells, data mining, model organisms, and omics technologies;
2) Developing novel stem cell/immune cell therapies and gene therapies;
3) Mesenchymal stem cells and extracellular vesicles;
4) Translational research and standardized production of cell and gene therapies.
Postdoctoral positions are available for candidates with a background in cell biology, immunology, computational biology, molecular biology, etc. Applicants should send their CV (PDF required) to sh.ou@siat.ac.cn, with the subject specifying "Application Position - School Name - Major - Full Name."