
Many diseases exhibit sex bias in their occurrence and development, but the mechanisms remain unclear. Previous studies have suggested that stem cell sex maintenance and disorder may be a new mechanism for disease sex bias. How stem cells coordinate intra- and extracellular signals to maintain sex characteristics and thus tissue homeostasis is a key issue. Although there has been extensive reporting on the mechanisms of sex determination and the impact of somatic cells on germ cells during embryonic development, the molecular mechanisms underlying the maintenance of adult stem cell sex identity and the related signaling between somatic and germ cells are largely unexplored.
On June 22, 2024, the team led by Ma Qing from the Institute of Synthetic Biology of the SIAT and Shenzhen Institute of Synthetic Biology, and the team led by Xu Jin from the School of Life Sciences, Sun Yat-sen University, published the latest research findings titled "Soma-Germline Communication Drives Sex Maintenance in the Drosophila Testis" online in the journal National Science Review.

Screenshot of the article online
Link:https://doi.org/10.1093/nsr/nwae215
This study utilized a model of male-specific reproductive system tumors caused by sex transformation in Drosophila testis stem cells, and combined scRNA-Seq and CUT&Tag technologies to demonstrate that the maintenance of adult Drosophila somatic cyst stem cells (CySCs) sex is mediated through the non-coding mutation of the Chinmo protein, which regulates the conserved sex determination pathway and insulin signaling pathway. The research elucidated the molecular characteristics of the feminization of Drosophila testis somatic stem cells at the single-cell level due to the non-coding mutation of the "sex switch" Chinmo, revealing the signal transduction mechanism whereby abnormal somatic cell-germ cell communication leads to gender disorder and tumorigenesis in germ cells. This study highlights the potential importance of stem cell sex instability as a new mechanism leading to reproductive system tumors and provides new insights into understanding the complex mechanisms behind tumorigenesis and infertility.
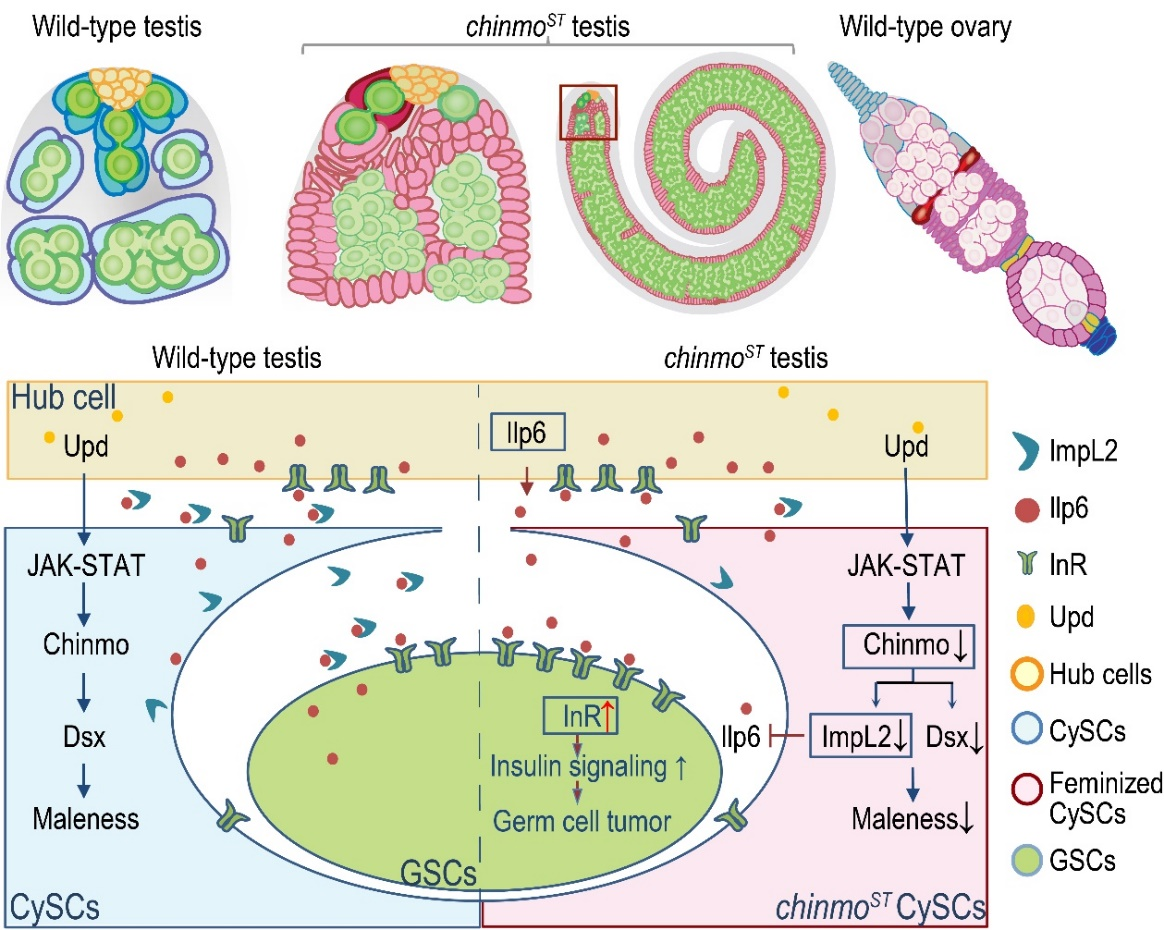
Figure 1: The "sex switch" Chinmo is regulated by the JAK-STAT signaling pathway, directly acting on the male sex determination factor DsxM (a gene in Drosophila homologous to the mammalian sex determination factor DMRT1) and insulin signaling pathway factors to maintain the male identity of the CySC lineage and promote normal spermatogenesis.
In many organisms, cell sex identity is established during embryonic development and was previously considered immutable. However, recent studies have found that the sex of somatic stem cells in adult gonads can also change, suggesting that the sex identity of somatic cells needs to be maintained in adulthood or after birth. So how do adult (stem) cells maintain their sex identity, and how do somatic cells interact with germ cells to maintain normal gametogenesis? The authors previously reported a Drosophila testis mutant phenotype in which somatic stem cells undergo sex transformation in adulthood, providing a suitable model for studying somatic cell sex maintenance and somatic cell-germ cell communication. The authors previously discovered that non-coding mutations in the key effector of the JAK-STAT signaling pathway and the "sex switch" Chronologically inappropriate morphogenesis (Chinmo) induced gender reversal in CySCs, leading to the occurrence of male-biased tumors. Due to the limited number of sex-specific somatic cell markers, it is impossible to comprehensively analyze the lineage trajectory of somatic stem cells undergoing transdifferentiation in real-time and the molecular characteristics of different somatic and germ cells. Moreover, has the signal communication between Chinmo-induced "sex-transformed" somatic stem cells and the "male" germ cells they are supposed to support changed?

Figure 2: Schematic diagrams of wild-type testes, chinmoST mutant testes, and wild-type ovaries, as well as staining images of wild-type and chinmoST mutant testes
To address the above questions, the authors performed single-cell RNA sequencing (scRNA-seq) on testes at multiple developmental stages of adult Drosophila to characterize the transcriptome landscapes of wild-type and chinmoST mutant testes. The authors identified cell populations unique to chinmoST and detected transcriptomic changes corresponding to the phenotype. By comparing the cell-cell communication networks between wild-type and chinmoST mutant testes, it was found that several somatic cell-germ cell signaling pathways, including the insulin signaling pathway, were disrupted in the chinmoST mutant testes, with notably enhanced signaling from feminized CySCs to germline stem cells (GSCs). Chinmo CUT&Tag (Cleavage Under Targets and Tagmentation) assay analysis revealed that Chinmo directly regulates two male sex determination factors, doublesex (dsx) and fruitless (fru), as well as regulatory factors of the insulin signaling pathway. Further genetic experiments in Drosophila confirmed that part of the observed gametogenesis defects in chinmoST mutant testes was due to the disruption of insulin pathway signaling. In summary, the study shows that somatic cell sex maintenance is facilitated by a conserved sex determination pathway and insulin signaling pathway mediated by Chinmo, which together promote normal gametogenesis.
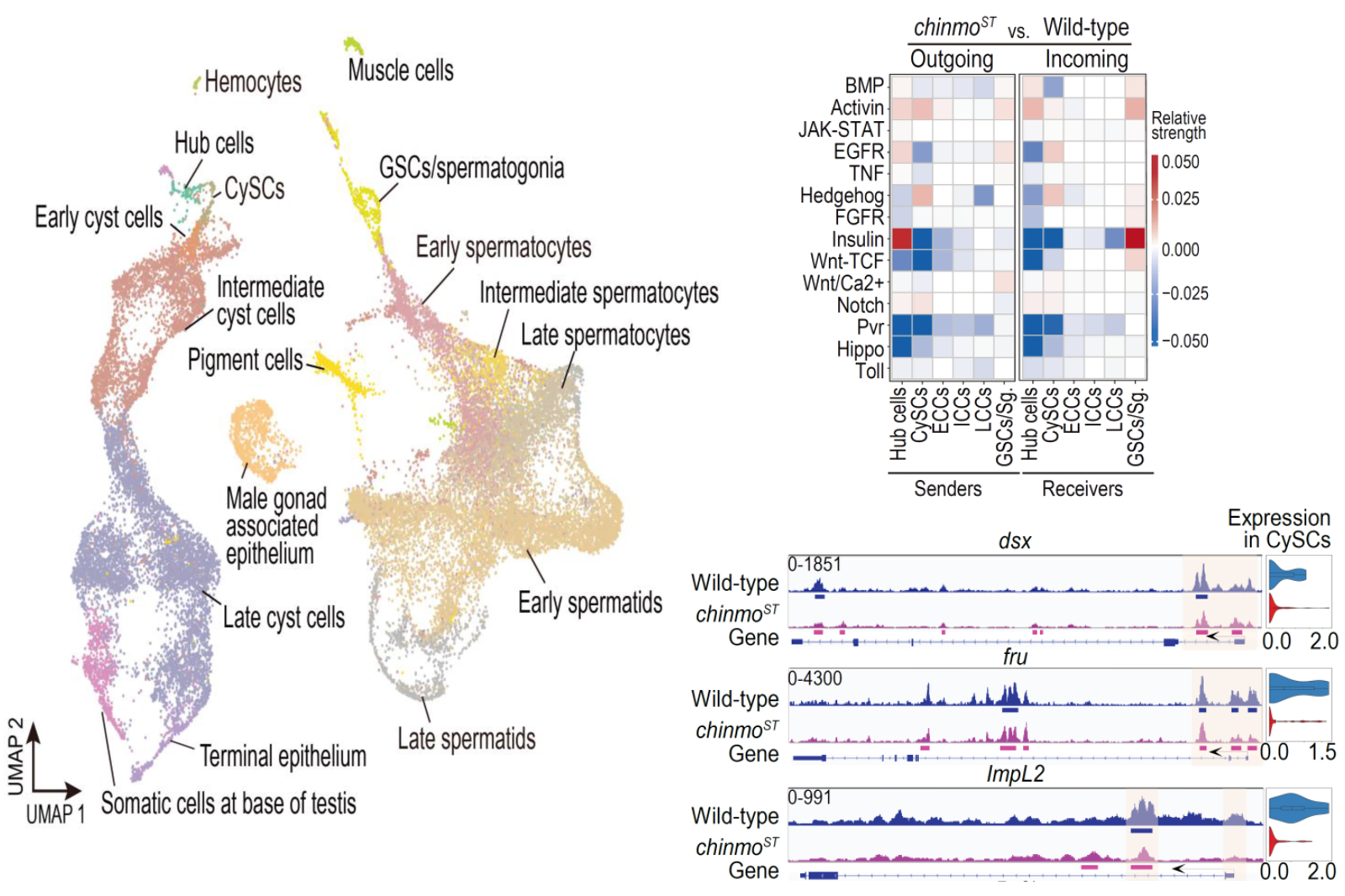
Figure 3: Single-cell data diagram of adult Drosophila testes, signaling between testicular cells, and examples of Chinmo target genes
This discovery provides new insights into the complex mechanisms of somatic cell sex maintenance and somatic cell-germ cell communication at the single-cell level, offering a new perspective for understanding the complex mechanisms behind infertility. It emphasizes the direct link between the instability of stem cell sex maintenance and the occurrence of testicular tumors and impaired fertility. Additionally, the authors previously found that the "sex switch" Chinmo can be regulated by some non-coding elements, playing a role in regulating stem cell sex and the occurrence of reproductive system tumors in the Drosophila germline center. This suggests the importance of non-coding regulation in stem cell sex maintenance and the development of sex-biased diseases. More importantly, many diseases show significant sex bias, such as autoimmune diseases, neurological disorders, cardiovascular diseases, infectious diseases (including COVID-19), and various cancers. Traditional disease treatment methods often overlook gender factors, sometimes leading to gender-specific responses to treatment outcomes. A comprehensive analysis of the molecular mechanisms underlying stem cell sex maintenance and the mediated intercellular interactions could provide new targets for the prevention and treatment of sex-biased diseases.
The co-corresponding authors of this paper are Ma Qing, a researcher with the SIAT, and Xu Jin, a professor at the School of Life Sciences, Sun Yat-sen University. Zhang Rui, an assistant researcher with the SIAT, and Shi Peiyu(former master's student at Sun Yat-sen University) are the co-first authors. This study was supported by funding from the National Key R&D Program of China, the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Guangdong Provincial Key Laboratory of Synthetic Genomics, and the Shenzhen Key Laboratory for Synthetic Genomics.

PI and Research Team: Ma Qing, a researcher with the Institute of Synthetic Biology of the SIAT, and doctoral supervisor. She has been selected for national-level talent programs and is the lead scientist of the National Key R&D Program's Youth Project in Synthetic Biology. The main research directions of her group include the functions and mechanisms of non-coding regions of the genome and non-coding RNAs in stem cell differentiation and epigenetic regulation, as well as the discovery and development of synthetic biology tools for non-coding RNAs. Her research achievements have been published as first or corresponding author in international journals such as Developmental Cell, eLife, Development, Genome Biology, and National Science Review.
The research team is always open to postdoctoral researchers and graduate students. Laboratory homepage: http://isynbio.siat.ac.cn/qingmalab/

PI and Research Team: Xu Jin, a professor with the School of Life Sciences, Sun Yat-sen University, and doctoral supervisor. She was recruited through Sun Yat-sen University's "Hundred Talents Program" in 2019 and has been selected for national-level talent programs. She is committed to studying regulatory mechanisms at multiple levels, including epigenetic modifications, transcriptional regulation, and post-transcriptional regulation with computational biology and single-cell multiomics technologies, aiming to elucidate the mechanisms and evolutionary processes underlying gene expression regulation. Her research achievements have been published as first or corresponding author in international journals such as Cell, Nature Genetics, Neuron, and eLife.
Laboratory homepage: https://lifesciences.sysu.edu.cn/teachersprofessor/2760
The two research teams have maintained close collaboration in the field of sex difference regulation. They jointly recruit postdoctoral researchers with expertise in bioinformatics or molecular biology, offering benefits from either institution and shared access to both teams' research resources. Interested applicants are invited to send their CV by email to qing.ma@siat.ac.cn or xujin7@mail.sysu.edu.cn.