
Solar energy is a typical clean energy source, and the development of green synthesis pathways driven by optical energy for high-value chemicals can help promote sustainable social development. Semiconductor-microbe hybrid systems (semi-artificial photosynthetic systems) combine the broad-spectrum light absorption characteristics of semiconductors with the high selectivity of microbial catalysis, offering the potential to efficiently convert solar energy into various forms of chemical energy. This has attracted widespread attention in recent years. Currently, researchers generally use semiconductor materials such as CdS, TiO2, and quantum dots along with microbe. However, there are still challenges including complex system construction processes, poor biocompatibility, low charge transfer efficiency, and unclear electron transfer mechanisms at the material-microbe interface, which require further exploration to enrich theoretical development. In recent years, porphyrin-based metal-organic frameworks (MOFs) have become a focus due to their high catalytic activity and adjustable structures. However, compared with other semiconductor materials, their application as light-harvesting modules in semi-artificial photosynthetic systems remains limited and is worth further exploring.
On April 25, 2024, the team led by Wang Bo from the Institute of Synthetic Biology of SIAT, in collaboration with the team led by Hou Yanping from the College of Resources and Environmental Materials, Guangxi University, published a research paper titled "A Self-Assembled MOF-Escherichia Coli Hybrid System for Light-Driven Fuels and Valuable Chemicals Synthesis" in the journal Advanced Science. This work constructed a self-assembled hybrid system using metal-organic framework (PCN-222) and Escherichia coli. Driven by optical energy, the hydrogen production of the hybrid system based on wild-type E. coli was 2.9 times that of the pure bacterial group, and the lysine production of the engineering E. coli-based hybrid system was 4.3 times that of the pure bacterial group, achieving light-driven synthesis of hydrogen and high-value chemicals. The authors studied the electron transfer mechanism at the material-microbe interface and demonstrated through photoelectrochemical testing that the hybrid system could efficiently capture light and transfer photogenerated electrons into the organism. Finally, they elucidated the mechanism related to PCN-222-E. coli light-driven chemical production. This work lays an important foundation for research on light-driven biosynthesis based on E. coli semi-artificial photosynthetic systems.

Screenshot of the article
Link: https://doi.org/10.1002/advs.202308597
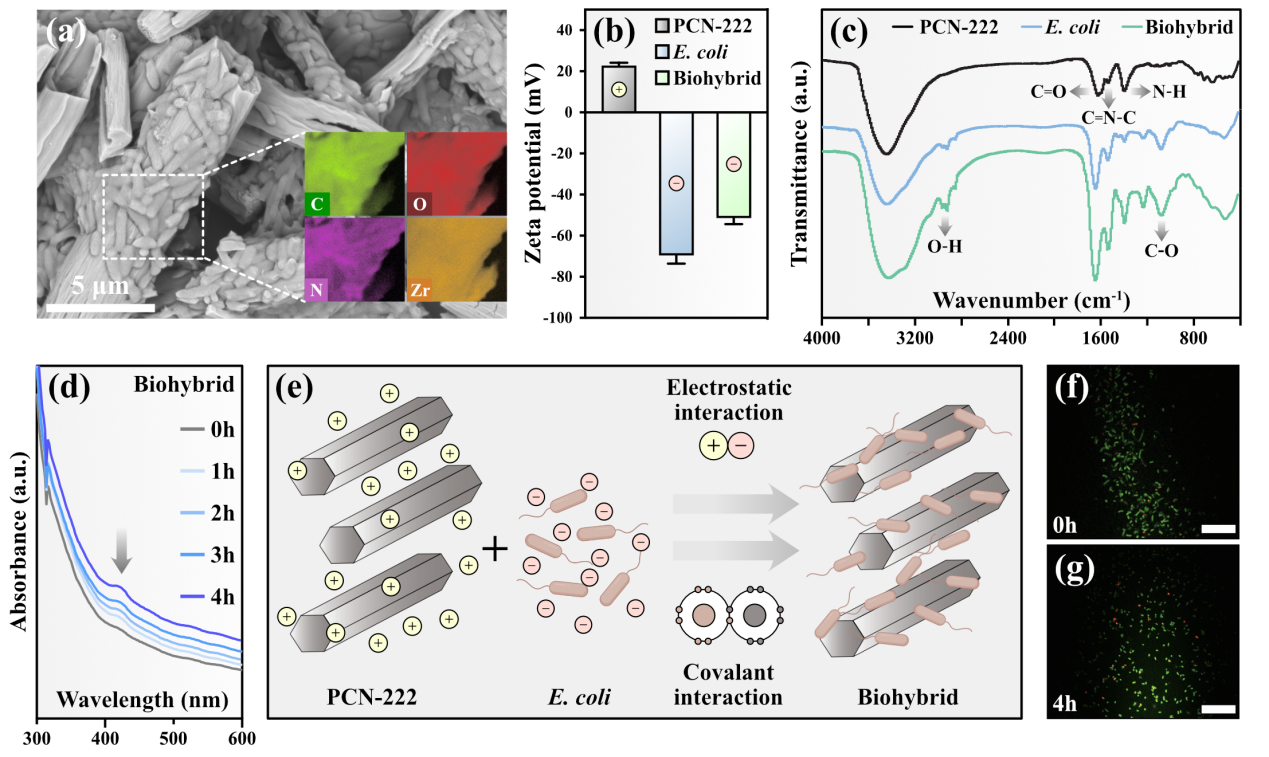
Figure 1. Characterization of the Metal-Organic Framework PCN-222-E. coli Hybrid
In recent years, MOF materials constructed based on zirconium-oxo clusters have been widely used in the field of photocatalysis research. Due to the high light capture efficiency and catalytic activity of MOF materials containing porphyrin or metalloporphyrin, the authors first synthesized a positively charged zirconium-based porphyrin metal-organic framework, PCN-222, and conducted relevant characterization tests on its physical structure. By observing the surface morphology of the hybrid through electron microscopy, analyzing surface potential, testing infrared spectra, and absorption spectra, it was found that the PCN-222 and negatively charged Escherichia coli hybrid was successfully constructed through electrostatic adsorption and covalent interactions.

Figure 2. Photocatalytic Hydrogen Production and Synthesis of High-Value Chemicals by the Metal-Organic Framework PCN-222-E. coli Hybrid
To verify that PCN-222 can act as an electron donor for Escherichia coli, the authors first constructed a hybrid using wild-type E. coli MG 1655, which has hydrogen production capability, combined with PCN-222. This system achieved efficient hydrogen production under illumination, confirming that visible light irradiation significantly promotes intracellular energy supply to E. coli by PCN-222. Subsequently, through genetic engineering, the lysine synthesis pathway was overexpressed in E. coli, and the performance of lysine synthesis in darkness and under illumination by the hybrid was studied. The performance of the PCN-222-E. coli hybrid system in photocatalytic lysine production was optimized across four dimensions: time gradient, the timing of material-E. coli hybridization, material concentration, and light intensity.

Figure 3. Mechanisms of Electron Transfer at the Material-Microbe Interface
After identifying the optimal conditions for photocatalytic lysine production, the authors focused on elucidating the electron transfer mechanism at the material-microbe interface in the hybrid. By testing the photoelectrochemical characteristics of pure bacteria and the hybrid, it was found that the hybrid has the ability to efficiently capture light and transfer electrons into the organism. To further confirm that PCN-222 can effectively transfer the captured light energy to E. coli, the authors used photoluminescence spectroscopy (PL) to study the electron transfer at the material-microbe interface. No significant fluorescence signal was detected inside E. coli cells, while the PL intensity of the hybrid was significantly lower than that of PCN-222 alone. This result indicates that in the hybrid, PCN-222 acts as an electron donor and E. coli as an electron acceptor, effectively reducing the electron-hole recombination rate of PCN-222. Subsequently, time-resolved photoluminescence spectroscopy (TRPL) was used to dynamically reveal the interfacial electron transfer during the photoreaction process. It was found that the fluorescence lifetime of the hybrid was shorter than that of PCN-222 alone, indicating that the photogenerated electrons from PCN-222 could be quickly transferred to E. coli, consistent with the results of the PL tests.
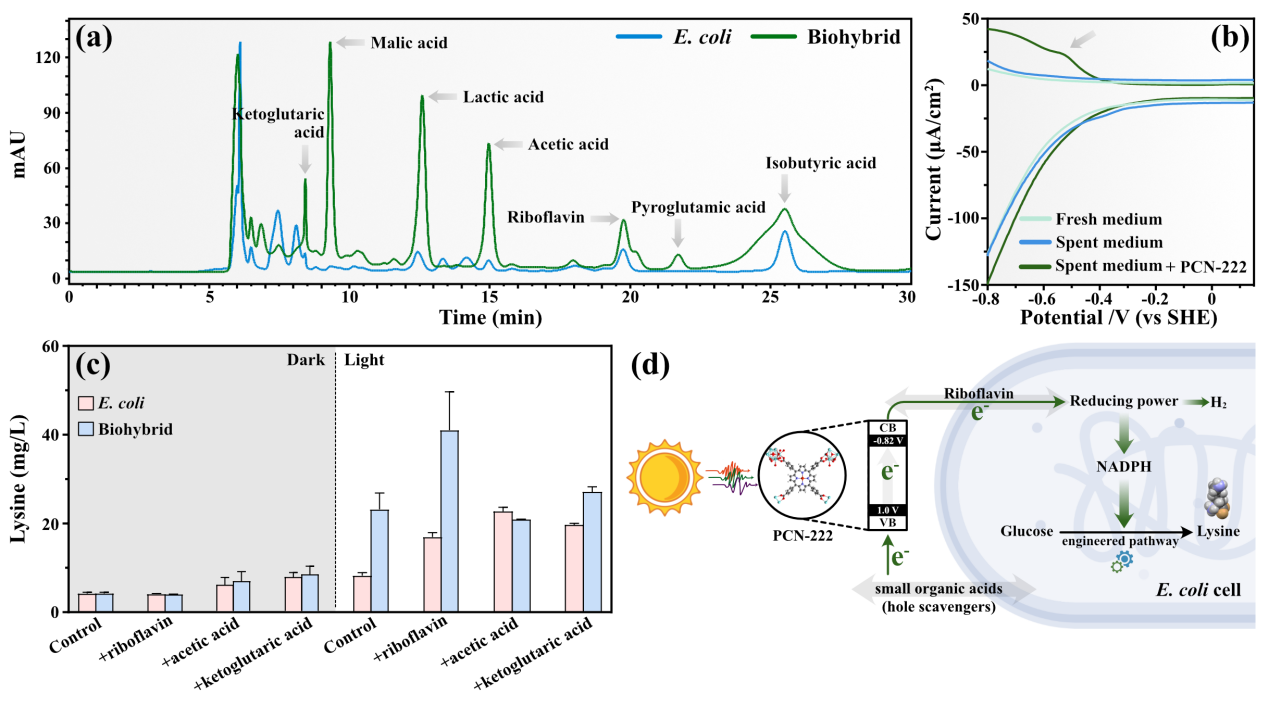
Figure 4. Mechanisms of Light-driven Hydrogen Production and High-Value Chemical Synthesis by the Hybrid
It is reported that endogenous redox substances secreted by Escherichia coli can act as electron transfer mediators, thereby enhancing the electron transfer rate at the interface. Therefore, this paper infers that redox mediators may contribute to photoelectron transmembrane transfer by the bacteria. After analyzing the supernatant of the hybrid post-reaction using high-performance liquid chromatography (HPLC) and differential pulse voltammetry (DPV), it was found that electrons could be transferred into the cell through small molecular organic acids and flavins as redox substances. Combining the role of PCN-222 in biological hydrogen production with the research results on electron transfer in the hybrid, this paper proposes a mechanism for the photocatalytic production of hydrogen and high-value chemicals by the PCN-222-Escherichia coli hybrid system, as shown in Figure 4-d. Due to electrostatic interactions, the positively charged PCN-222 self-assembles with the negatively charged Escherichia coli to form a semi-artificial photosynthetic system. Under moderate visible light irradiation, PCN-222, with its excellent light-harvesting performance, generates a large number of photogenerated electrons, which are transferred into Escherichia coli through redox mediators. In terms of electron transfer, the photogenerated electrons from PCN-222 stimulate the secretion of endogenous flavins, aiding in electron transmembrane transport. Meanwhile, some small molecular organic acids can act as hole scavengers for PCN-222, promoting electron migration. Thus, under illumination, the photogenerated electrons acquired by Escherichia coli enhance the intracellular reducing power level, leading to the conversion of excess reducing power into hydrogen and promoting lysine synthesis, achieving efficient photodriven synthesis of hydrogen and lysine.
This study was supported by funding from the Key Research and Development Program of Synthetic Biology of the Ministry of Science and Technology, the National Natural Science Foundation of China, the Guangdong Provincial Key R&D Program, and the Shenzhen Institute of Synthetic Biology.
PI and Research Group:
Wang Bo, Associate Research Fellow, Ph.D. supervisor, Young Chief Scientist of the National Key R&D Program, member of the Chinese Academy of Sciences Youth Innovation Promotion Association. The team's research field is Material Synthetic Biology, with the main laboratory directions including the design and construction of artificial hybrid systems between semiconductor materials and microbial cell factories to achieve light-driven synthesis of target chemicals and elucidating the electron transfer mechanisms at the material-cell interface and the intracellular energy conversion mechanisms. In recent years, as the first author and corresponding author (including co-author), he has published 20 articles in journals such as Advanced Science, Angewandte, Advanced Energy Materials, Energy & Environmental Science, and Nano Energy. He is always open to postdoctoral researchers and joint doctoral students interested in related fields. Please feel free to contact him at bo.wang@siat.ac.cn.