
Recently, Ma Yingxin, a researcher with the Institute of Synthetic Biology, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, and Dai Junbiao, a researcher with the Shenzhen Institute of Agricultural Genome, Chinese Academy of Agricultural Sciences, published a review entitled “When Synthetic Biology Meets Medicine” in the new academic journal Life Medicine.
The review focuses on the thinking revolution of synthetic biology tools for medical applications, divided into three stages: acceleration, upgrading and innovation, and gives a comprehensive overview of the role of synthetic biology in drug discovery and production, diagnosis and treatment of diseases in recent years.
Feng Yuge, a PhD candidate with Shenzhen Institute of Advanced Technology, is the first author, while researcher Ma Yingxin with Shenzhen Institute of Advanced Technology, and researcher Dai Junbiao with Shenzhen Institute of Agricultural Genomics are the corresponding authors of the article.
Screenshot of the article
Link:https://doi.org/10.1093/lifemedi/lnae010
In recent years, the Covid-19 pandemic and other pandemics, including the Zika virus and Ebola viruses, have shown an alarming speed and scale of the global spread of infectious diseases. The rapid growth of non-communicable diseases such as heart disease, diabetes and cancers also puts great pressure on the healthcare system. While drug development, diagnosis and treatment progress of diseases still lag behind the emergence of diseases, many urgent clinical needs remain unaddressed. New technologies need to be explored to discover and develop biomedicine and biological therapies. Synthetic biology is a revolutionary field in modern medicine, which focuses on designing artificially engineered systems for specific purposes with modular systems, ranging from simple units such as enzymes and regulatory elements to complex modules through precise adjustment and mathematical combination, which has formed multiple genetic circuits. This review summarizes the latest advances in applications of synthetic biology in medicine, with a particular focus on its role in drug discovery and production as well as diagnosis and treatment of various diseases.
What does synthetic biology mean for medicine? Starting with the enabling technology of synthetic biology, this review vividly explains the thinking changes and applications of synthetic biology in three different stages: the acceleration of production, the update of rapid detection of diseases, and the innovation of accurate treatment of diseases.
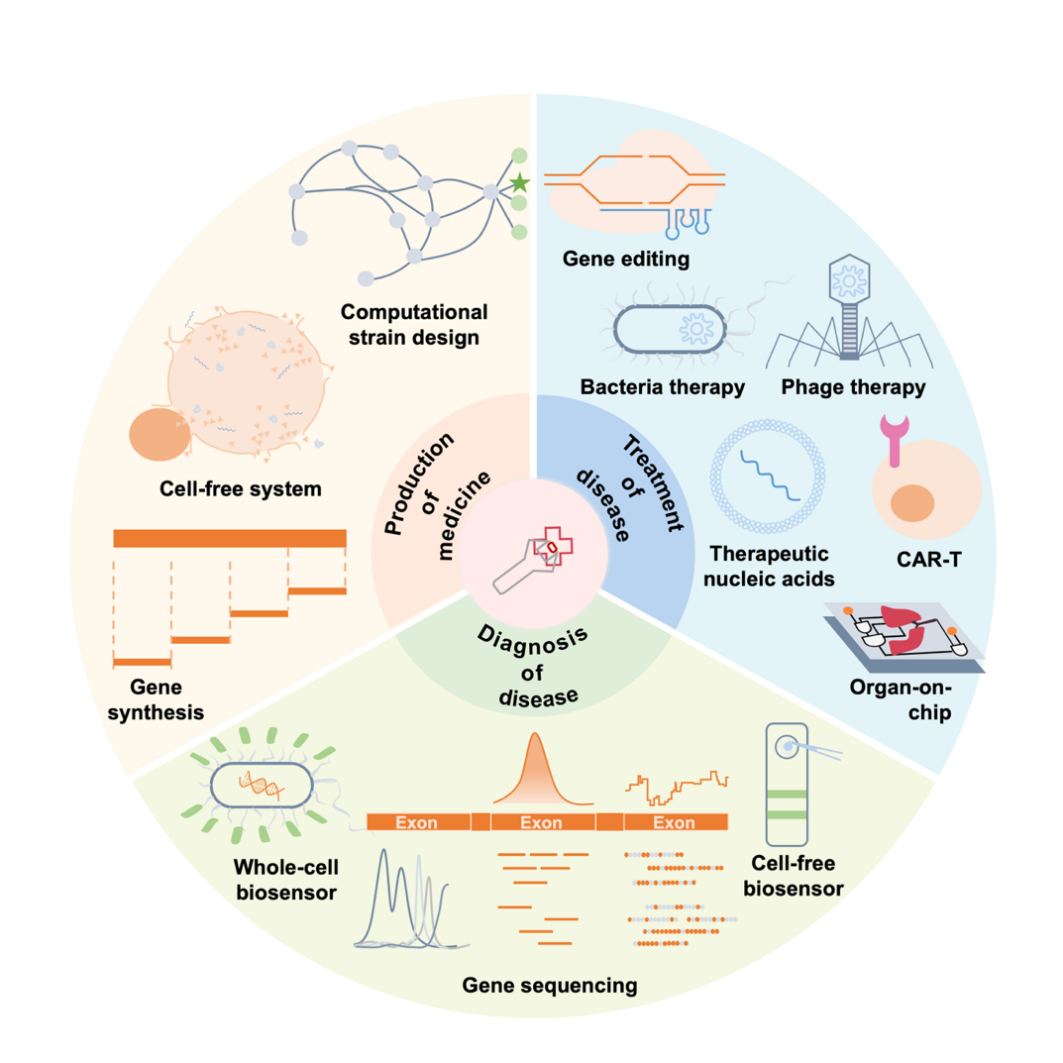
Figure 1 When synthetic biology meets medicine
What is the disruption of synthetic biology ideas? This review highlights the concept of modularity in synthetic biology. Using the “input” and “output” in a broader sense, and DNA as the coding elements, according to the existing biological knowledge and the needs of people, multiple genes or biomolecules are coupled and assembled into a biological module with certain functions, and eventually the multiple function modules are coupled into a system, which is used to achieve biological functions that do not exist in nature, but are needed by humankind.
1 Synthetic biology accelerates the production of drug molecules
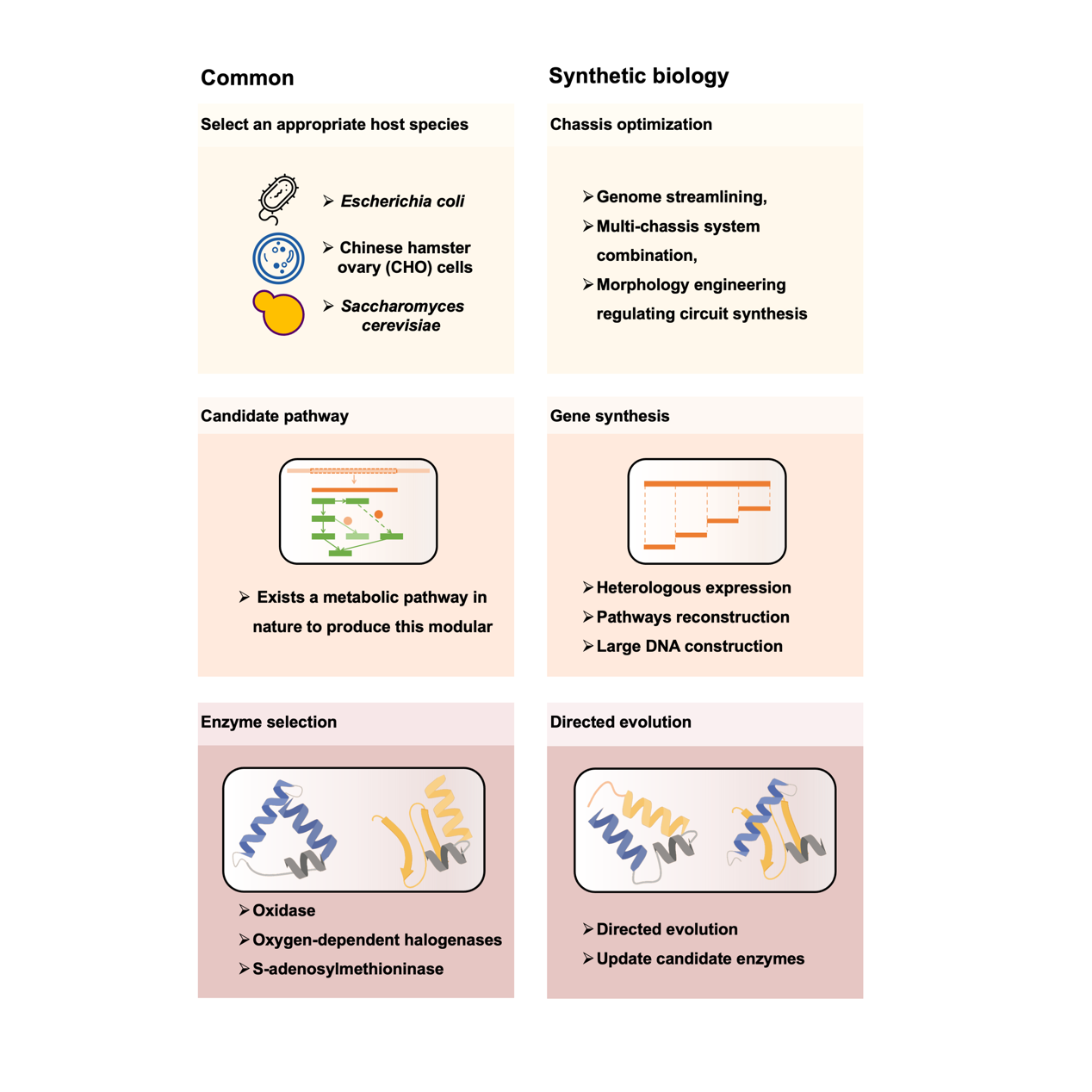
Figure 2 Production of synthetic drugs
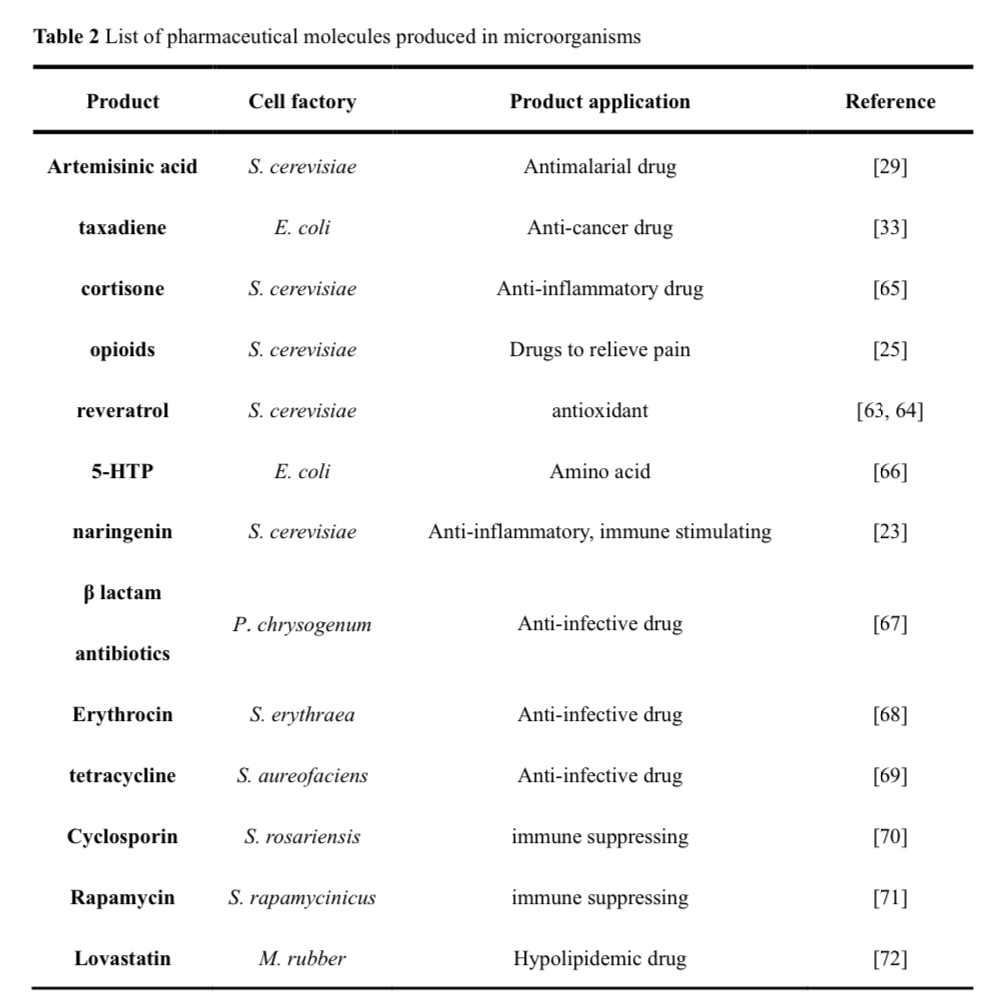
The process of drug molecules biomanufacturing involves three key steps: the operation and generation of chassis cells, the construction and integration of molecular synthesis pathways, and the artificial regulation and enhancement of enzymes in the metabolic network. In producing specific drug molecules in cell factories, the “operable engineered chassis” is often adopted, with prominent examples including E. coli, Saccharomyces cerevisiae, and Chinese hamster ovary cells. The development of synthetic biology has greatly accelerated this process, such as the construction of artificial cells with minimized genomes as a more controllable chassis, or the inclusion of non-standard amino acids into metabolic remodeling, and the application of new enzymes. A lot of successful applications have been achieved, including laboratory-scale Cannabinoids, monoterpene indao alkaloids (MIAs), unnatural amino acid drugs, and plant-scale Amorphadiene and Ginsenosides.
Besides, the advanced synthetic biology tool of cell-free systems is playing an increasingly important role in biopharmaceutical production and development, which flexibly and precisely control peptides without cellular and membrane constraints, and concentrate the use of energy without a highly evolved internal system. Over the years, cell-free systems have been applied in the production of various biological drugs, such as antibody derivatives, antibody-drug couplers, cytokines, vaccines, membrane proteins, metalloproteins, viral proteins, and anti-microbial peptides.
2 Synthetic biology upgrades the disease diagnosis
Traditional diagnostic methods such as imaging and biochemical testing may not accurately diagnose all types of disease, especially in early stages or with atypical symptoms. However, with the rapid development of DNA and RNA sequencing technologies, it enables precise identification of diseases through the direct extraction of genetic material samples from patients and assessment of disease risk based on genotype. Sequencing technology also shows high sensitivity and specificity in the diagnosis of pathogens, which can be used to quickly detect and fully identify viruses, bacteria, fungi and parasites. If this is true for diseases with priori knowledge, up to 60% of infectious diseases remain unknown. For emerging, rare, or challenging infectious diseases, sequencing technologies offer a great advantage absent in traditional diagnostics.
The pathogen genomic information obtained from analysis allows us to understand its gene characteristics, such as drug sensitivity and resistance, and predict its evolution and mutational trends, which is helpful in the development of effective public health strategies. During the pandemic of Covid-19, sequencing technology has played an important role. Its another important role in health and environmental monitoring should be whole-cell and cell-free sensors.
From traditional whole-cell activity identification to the utilization of natural disease biology and reconstructed enzyme function, the biosensors have achieved increasingly complex clinical performance characterized by sensitivity, specificity, portability, and sustainability. The gene detection circuit comprises three types of components: sensing, signal processing, and output elements. Whole-cell sensors refer to the cells containing transducer elements that are typically composed of transcription factors that have gene expression for the recognition and induction of analytes. The signal processing elements convert the induced signal into measurable signal to achieve the analyte detection. These signals often cover amplifiers, feedback loops, or logic gates. Many reporter proteins that produce fluorescence, color, electrons, or gas can serve as output elements. DNA recombination has made a significant contribution to it.
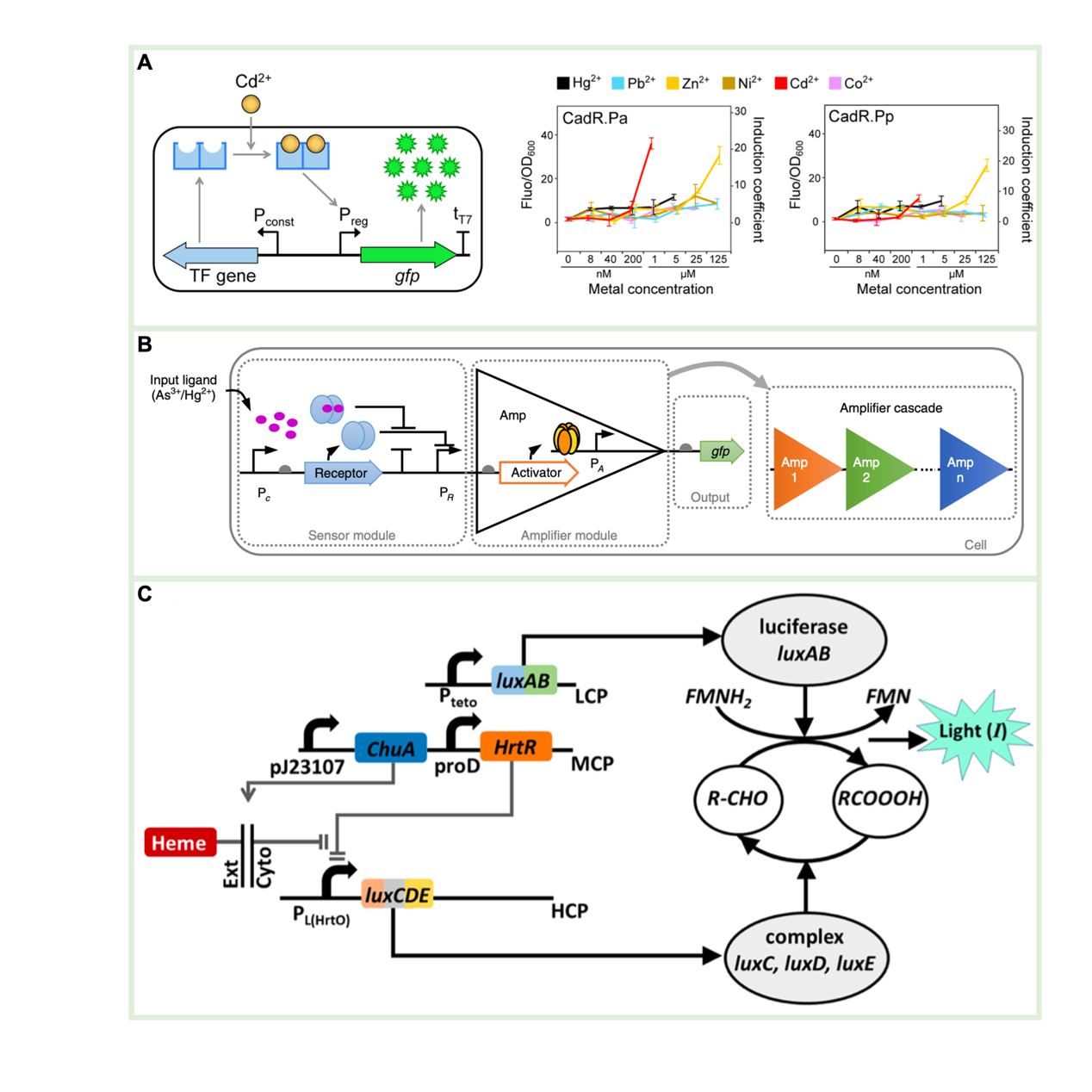 Figure 3 Whole-cell sensor
Figure 3 Whole-cell sensor
The cell-free sensor is a trace substance detection tool designed based on in vitro cell-free systems, which has the advantages of removing the transmembrane transport barriers and overcoming the biosafety and nutrient limitations associated with cell storage. It has received wide attention from the fields of rapid detection, including metal ions, quorum-sensing molecules, antibiotics, and viruses. As highlighted in this review are the latest achievements in improving the sensitivity and portability of cell-free sensors. These important fields include activation and repression of transcription factors, Toehold switching and CRISPR-Cas recognition.
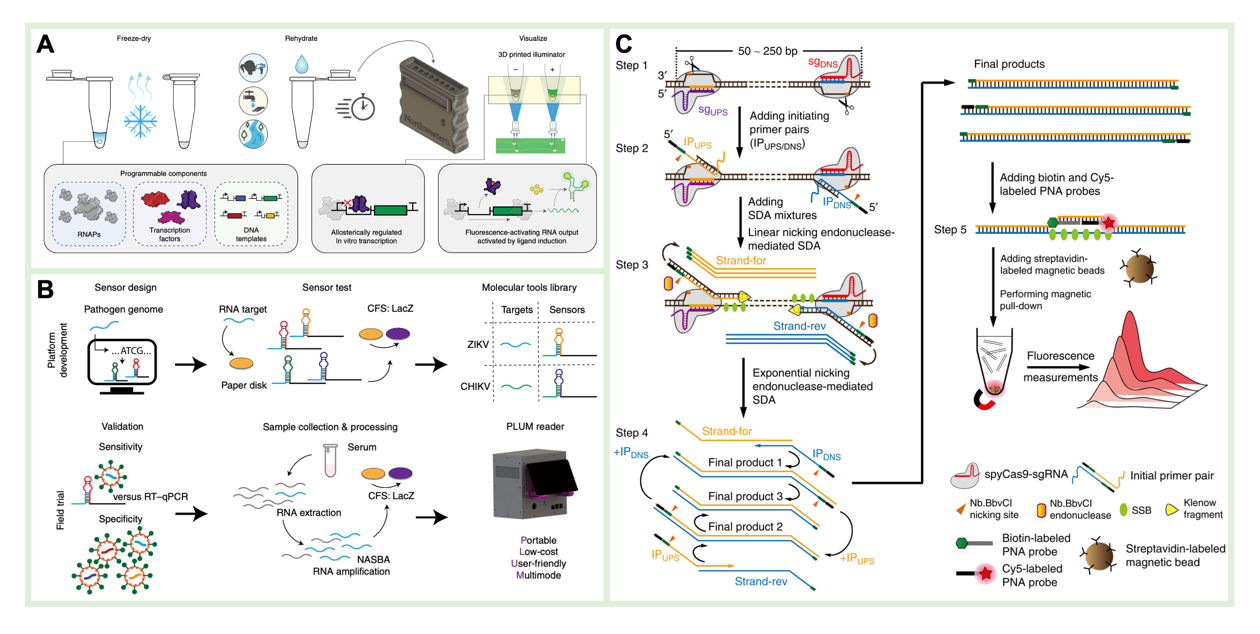
Figure 4 Cell-free sensor
3 Synthetic biology revolutionizes the treatment of diseases
The treatment of diseases is a typical challenge in the medical field. It takes two basic steps to address human body’s innate response to diseases: pinpointing the affected site and implementing effective treatments. The emergence of synthetic biology has enabled a thorough change to the treatment of diseases, which is reflected in a programmable paradigm, a concept embodied in every stage and responding to different intracellular and exogenous signals. Looking into the future, this review focuses on several pioneering strategies, including gene editing, therapeutic nucleic acid drugs, cell therapy, virus-based therapy, bacterial therapy, and organ engineering.
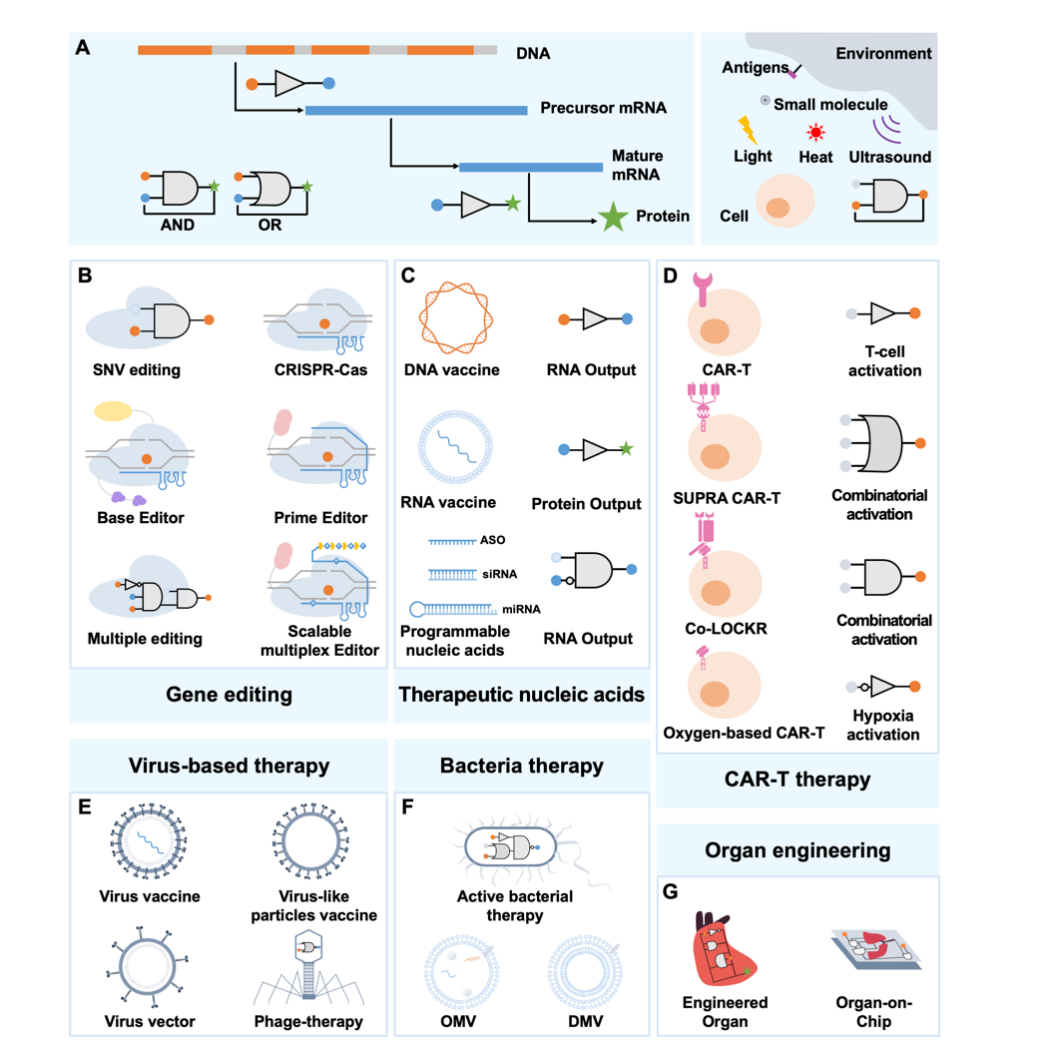
Figure Figure 5 Synthetic biology innovates the therapy of diseases
Gene editing is one of the most powerful and extensive tools in synthetic biology and has great potential in the next generation of gene therapy. This review focuses on the progress of treating genetic diseases with gene editing tools, focusing on CRISPR-Cas, base editing and primer editing [please visit the link: https://mp.weixin.qq.com/s/odnyFT7RkSTFPJcdB1YLBA], and their use in single-nucleotide-mutated diseases and complex diseases with multi-genetic mutations.
Therapeutic nucleic acids are a new generation of pharmaceutical technologies that are becoming a promising candidate in the biomedical field. They are modified RNA or DNA with different functions to treat the diseases at the gene level and achieve a lasting efficacy. Some treatment-related nucleic acid models have been developed, including DNA vaccines, RNA vaccines, and programmable molecules, such as mini RNA (miRNA), small interfering RNA (siRNA), and antisense oligonucleotides (ASO). These tools have been approved or are undergoing clinical trials.
Another established case clinically approved is CAR-T therapy, one of the most successful cases of engineered cell therapy. Cell therapy involves using modified or unmodified cells cultured in vitro, which are later fed back to human body. When combined with the target antigen, activation of the stimulatory and costimulatory domains promotes T cell proliferation and kills target cells. T cells equipped with the localization device “CAR” can specifically recognize tumor cells and release various effectors, exhibiting potent anticancer cytotoxicity. One of the unique advantages of CAR-T therapy is the ability to achieve genetically controlled circuits. CAR-T cells can be created more precisely when further amplified into artificial synthetic programs that recognize specific signals and perform specific functions. Synthetic biology opens up new possibilities for the design of engineered chimeric T cells with enhanced control, flexibility, and specificity. This review is an overview of two advanced strategies: autonomous control and external control. Immune cells engineered to express autonomous circuits can bind with signals from engineered immune cells or natural environments, including intracellular states, antigens, and the tumor microenvironment. Another key approach is to design cell therapies with different signals derived from external reagents, such as light, ultrasound, and small molecules. Autonomous control signals can be combined and deployed to address complex cancer conditions. Here, we focus on two advanced logic circuits: the SUPRA CAR system and the Co-LOCKR system. Additional solutions with external control circuits provide more reliable security. CAR-T therapies for five malignant tumors approved by the FDA show a promising efficacy and clinical anticancer cytotoxicity.
Virus-based therapeutic strategies also show promising prospects, including viral vaccines, viral vectors, and phage therapy. Viral vaccines have been proven to be one of the most effective therapeutic approaches in the COVID-19 pandemic. Compared with DNA and RNA vaccines mentioned above, virus-based vaccines are more mature, including inactivated, attenuated, and virus-like particles, which have been used to deal with a range of diseases, including poliomyelitis, influenza, SARS, Ebola, and smallpox. Advances in gene therapy have prompted the emergence of viral vectors for the delivery of therapeutic proteins and gene-edited fragments. Commonly used viral vectors include retroviruses, adenovirus, adeno-associated viruses, herpes simplex virus, and lentivirus for the delivery of monoclonal antibodies, anticoagulants, blood factors, enzymes, growth factors, hormones, and engineered proteins. Over the past two decades, more than 20 viral vector therapies have been approved, which are mainly applied in the treatment of cancers, monogenic diseases, and infectious diseases. Virus-based strategies also bring breakthroughs in anti-microbial resistance strategies. Phage therapy infects bacterial cells by attaching their tail ends to the bacterial cell wall and injecting their genome into the capsid (head). These phages can be classified into virulent phages and temperate phages. Both therapeutically engineered phages, and effective vectors for delivery, have been proven to have a high therapeutic potential. Since 2020, 29 clinical trials designing phage therapies have been initiated.
Besides phages, bacteria are another promising therapeutic platform. By re-editing a variety of bacteria, synthetic biology offers potential therapies for various diseases, including metabolic, gastrointestinal, and oncological diseases. Engineered microorganisms can be defined as general gene circuits capable of manual setting of input, manipulation, and output, and can be used as targeting vectors for specific pathogens or vectors for delivery of therapeutic molecules. Engineered therapeutic bacteria holds great promise in future tumor therapies. Bacterial materials are also a potential therapeutic tool. Concentrated bacterial membrane vesicles (BMV) have been used as vaccines or vaccine adjuvants for viral infections and cancers. These vesicles include membrane vesicles (MV) of Gram-positive bacteria, outer membrane vesicles (OMV) of Gram-negative bacteria, bilayer membrane vesicles, and protoplast-derived nanosized vesicles (PDNVs). Moreover, engineered bacterial communities demonstrate a smarter prospect in therapy. By integrating multiple bacteria into engineered consortia with complex metabolic interactions, it can further enable the complex cascade of multiple biomedical strategies, providing a promising direction for the future development of engineered bacterial therapy.
This review concludes with a discussion of organ and organoid therapies that have gained much attention in recent years. Germline engineering has been dedicated to developing organs, tissues, and cells for human transplantation. Its goal is not only to address organ defects, but also to perform multiple editing of organs to make them superior to natural human organs. The organoid chip provides a precise model that simulates the spatial characteristics and microenvironment of the physiopathology of the organs in human body. The replication of such in vivo microphysiological system provides a new option for disease studies and drug screening.
In conclusion, this review summarizes the shift from pharmacological or therapeutic nucleic acids to modular gene circuits and further to artificial living cell systems that may eventually extend to organs and reproductive system. This process covers current hotspots and promising medical applications in synthetic biology, including cell factories for drug production, biocatalysis, new drug discovery, gene circuits, gene editing and genome records for disease monitoring, and live probiotic therapies. Certain applications, such as commercial cell factories and CAR-T therapies, have significantly matured, and attracted attention from enthusiastic investors, while others, such as nucleic acid vaccines and viral vector delivery, have made substantial contributions to global pandemic management. The rise of synthetic biology in medical applications is now overwhelming. The growing biomedical dream, once deemed unattainable, is now being realized through the new frontiers of synthetic biology.
In the future, one of the long-standing scientific pursuits of synthetic biology seems to create biological intelligence. Significant progress has been made, setting precedents in the synthesis and assembly of encoded E. coli fragments, a complete small genome of genital mycobacteria, and a complete set of yeast chromosomes (Sc 2.0). Recent studies such as artificial prokaryotic systems, DNA storage, and genome writing further demonstrate the possibility of attaining this goal. These achievements open the door to redesign, innovation, and even beyond life itself, revolutionizing medicine.
Another important experimental pathway involves the further interdisciplinary integration, including computer-aided design (CAD), biological logic computing, and artificial intelligence (AI). The increasingly enhanced multidisciplinary integration with synthetic biology will undoubtedly usher in a new era of “innovative medicine of synthetic biology”.
Synthetic biology is gaining increasing attention in medical applications, demonstrating innovative technological collaboration between academia and industry. However, there are considerable challenges ahead, especially in terms of safety, bioethical and legal considerations. As medical therapies ultimately aim to address human health concerns, it has become critical to address these shortages and bottlenecks.
This review only reaches the surface of the enormous possibilities that may be brought by synthetic biology. Given the extraordinary progress made to date and the numerous ongoing efforts in medical applications, we anticipate that the black box of species structure and function will be opened in the future. This will lead to a leap from principle products to intelligent design in biology. Overall, synthetic biology marks the beginning of a new era in medicine.