
Recently, the research team led by Zhou Jiahai / Gu Yang with Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences published a research paper entitled “Enzymatic Photodecarboxylation on Secondary and Tertiary Carboxylic Acids” on the journal Org. Lett.. Through rational design and directed evolution of the photodecarboxylase FAP, this study developed a new type of photodecarboxylase, which achieved efficient photodecarboxylation on photodecarboxylic acids of secondary and tertiary non-natural substrates, and was applied to the chiral synthesis of drug molecular analogs.

Screenshot of the article
Link: https://pubs.acs.org/doi/10.1021/acs.orglett.3c03356
As the basic molecule in chemical synthesis and biotransformation, the green and efficient transformation of carboxylic acid has attracted important attention in the field of synthesis science. The traditional decarboxylation reaction requires harsh reaction conditions such as high temperature mediation, the introduction of activation group, and the involvement of equivalent metal. The carboxylation of carboxylic acid with mild conditions and good group compatibility can be developed to provide an important synthesis tool for the green synthesis and manufacturing based on carboxylic acids. Photocatalysis can use green renewable light energy and achieve challenging transformation under mild conditions through the activation of the photo-excited state; and enzymatic catalysis can use the rich secondary interaction of protein molecules and substrates to realize the selective transformation of complex molecules. Therefore, the development of a photoenzyme catalytic system is expected to achieve the highly selective transformation of carboxylic acid molecules under mild conditions.
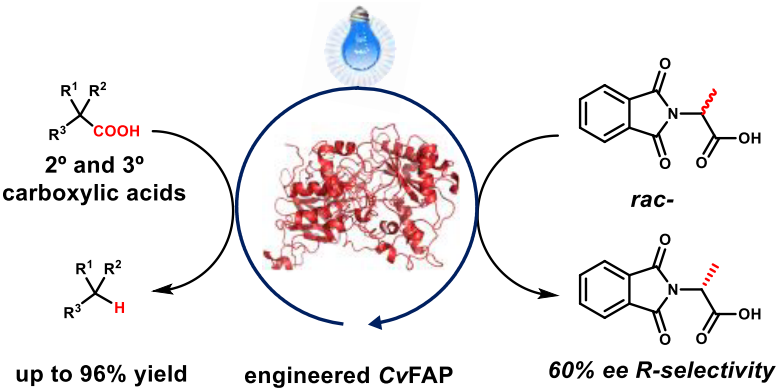
Figure 1 Transformation and application of photodecarboxylase
As a type of photoenzymes with photodecarboxylation activity, FAP can decarboxylate natural substrates such as oil and generate corresponding alkanes under blue-light induction, but the wild-type FAP has low reactivity for non-natural substrates with large resistance such as secondary and tertiary carboxylic acids, which limits its application scope. Through structure-based design and directed evolution of wild-type FAP, the team discovered the triple mutant containing Y466F / F469V / T131V, and achieved the photodecarboxylation of hindered carboxylic acids such as amantllic acid. Subsequently, in further optimization of the conditions for the enzymatic reaction, the team discovered that the highly stable lyophilized whole cells, easy to operate can be used as a catalyst to greatly improve the conversion rate of the reaction.
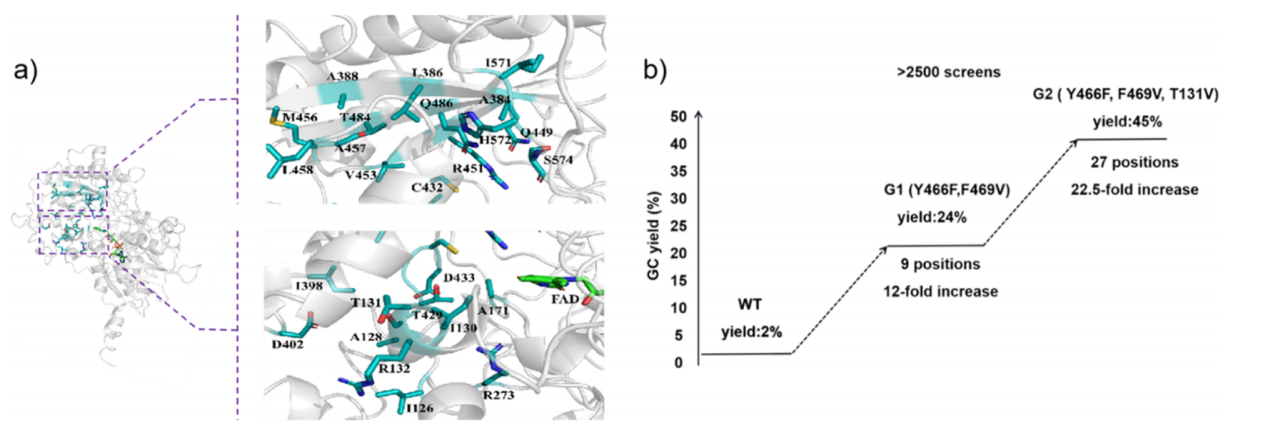
Figure 2 Rational design and directed evolution of photodecarboxylase
Under the optimal conditions, the research team studied the substrate applicability. The photoenzyme catalytic system has a broad spectrum and excellent group compatibility, and has a good conversion rate for a series of secondary and tertiary carboxylic acids, including substrates containing various functional groups such as ketone groups, lipid groups and amine groups. It is noteworthy that for important drug molecular intermediates such as heterocyclic carboxylic acids, the photoenzyme can also successfully achieve its photodecarboxylation conversion and chiral synthesis of racemic substrates, which proves that the photoenzyme can be used in the biosynthesis of drug molecules. Finally, through means of computations such as the molecular docking of the template substrate and the protein structure and kinetic simulation, the research team further explained that the distance between the carboxylate substrate and the photoreactive center FAD molecule was the key factor to determine the reaction rate, which provided theoretical guidance for the subsequent photoenzyme modification.
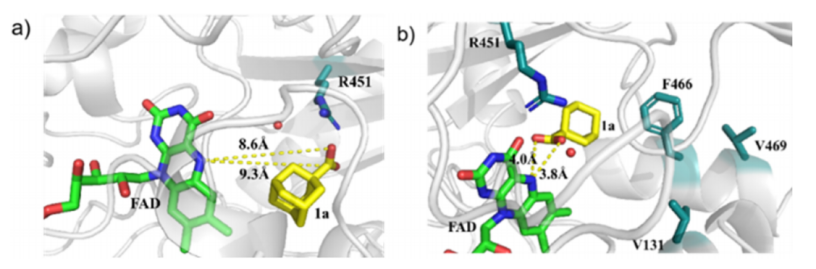
Figure 3 Theoretical simulation and catalytic mechanism analysis of photoproacararboxylase
In conclusion, this study discovered a type of tool enzymes that can catalyze secondary and tertiary photodecarboxylation of carboxylic acids, expanded the synthesis strategy of photoproenzyme-catalyzed biotransformation, and enriched the photocontrolled enzyme elements of synthetic biology.
The first author of this paper is Zheng Jie, a PhD student co-cultivated by the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences and Northwest Agriculture and Forestry University. The corresponding authors include professor Gao Jinming, researcher Zhou Jiahai and associate researcher Gu Yang with Shenzhen. Dr. Chen Zhuanglin with Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, also made an important contribution to the theoretical calculation part. This research was supported by the Key Research and Development Program of the Ministry of Science and Technology (2021YFF1200302,2019YFA09005000), National Natural Science Foundation of China (22201295,22077102), Shenzhen Science and Technology Innovation Commission (JCYJ20220818100804010, ZDSYS20210623091810032) and Shenzhen Institute of Synthetic Biology.