From equations to living cells
Recently, Prof. Fan Jin and his team from Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences achieved two major research breakthroughs in the field of quantitative synthetic biology, both of which were published in Nature Physics.
Adopting the core approach of "theory first," they combined mathematical modeling, experimental validation, and automation technology to successfully construct a frequency-decoding cell and uncover the laws governing the optimal transmission frequency of second-messenger signals (Figure 1). These advancements open new avenues for research on cellular regulatory mechanisms and applications in synthetic biology.
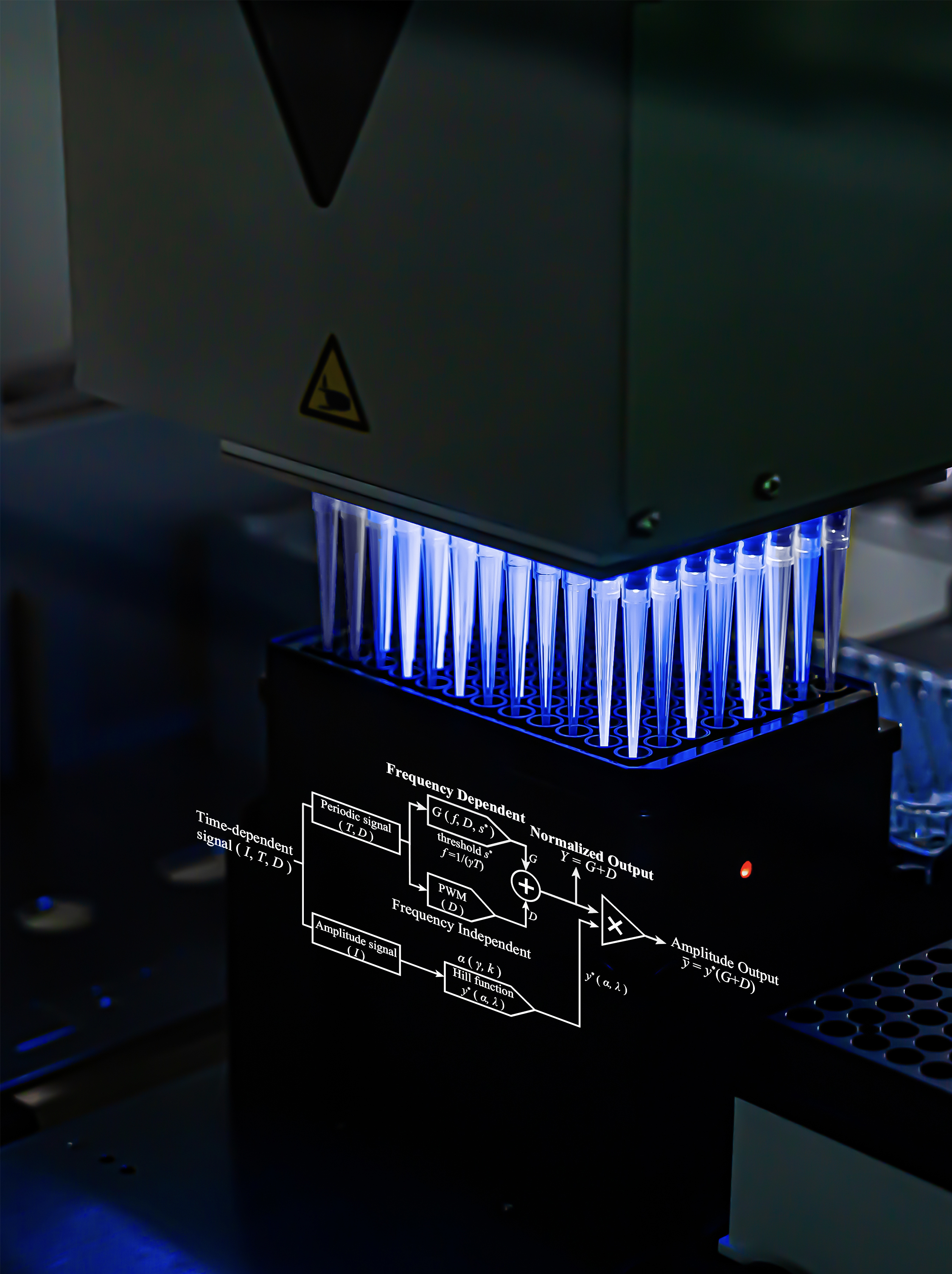
Figure 1 From equations to living cells.
In the first study, titled "Quantifying second-messenger information transmission in bacteria," the team similarly began with a theoretical breakthrough. In mid-2021, during a moment of reflection in their Xili office in Shenzhen, Fan Jin proposed a key insight: if second messengers carry information, there must exist an optimal frequency that maximizes reliable information transmission. By the evening of the same day, he had derived an analytical expression for this optimal frequency and unexpectedly discovered that near this optimal frequency, the most efficient encoding method is binary, with two states per cycle. This theoretical framework guided the team to establish a disciplined research pathway: analytical modeling first, followed by simulation, and culminating in experimental validation (Figure 2).
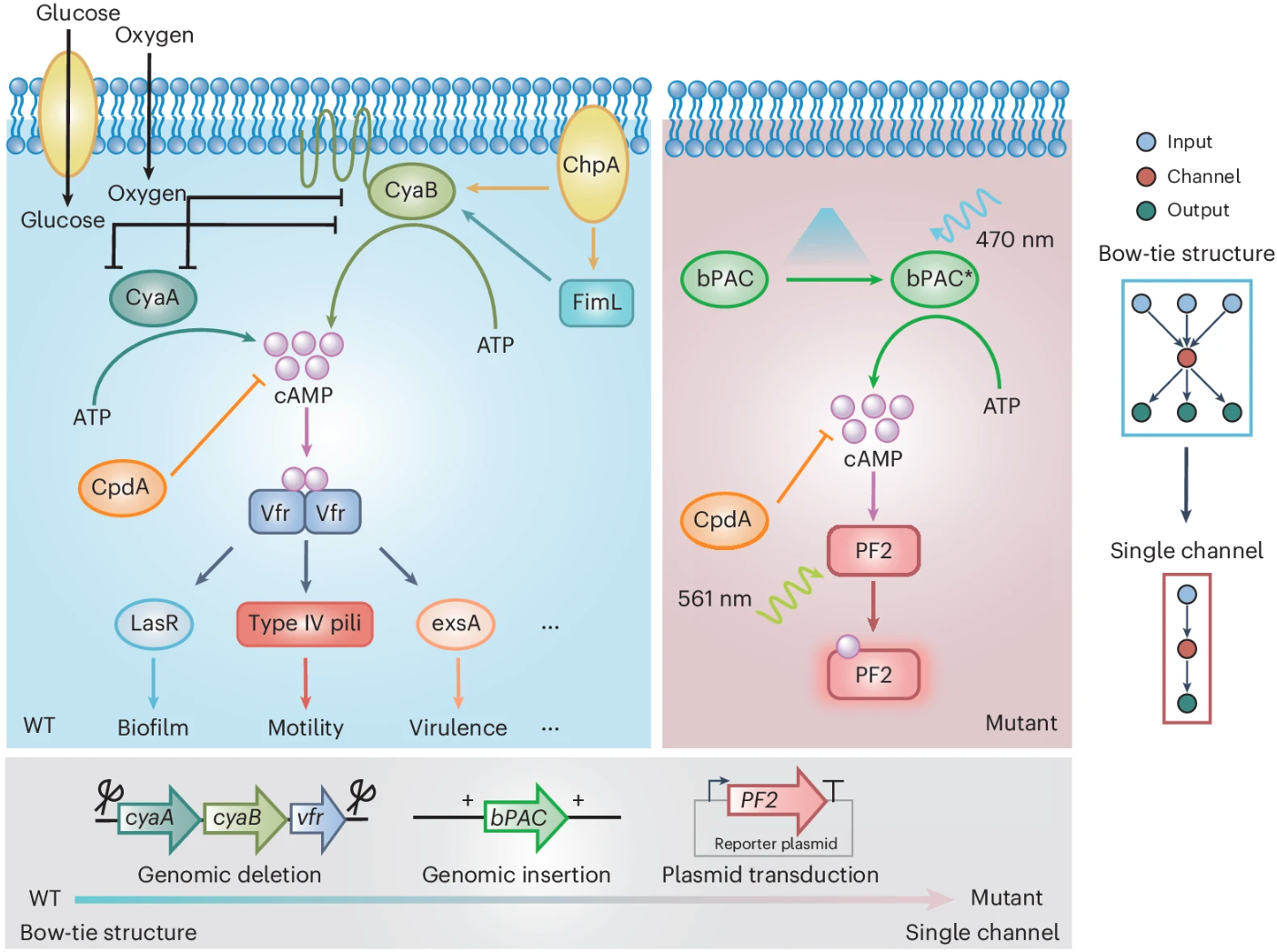
Figure 2 Engineering a synthetic circuit utilizing bPAC and PF2 for measuring information transmission via the cAMP pathway. Modified from the original paper.
To match the rigor of the theory, the team once again constructed a "clean" cyclic adenosine monophosphate (cAMP) signaling pathway in Pseudomonas aeruginosa: using a blue-light inducible adenylyl cyclase (bPAC) to synthesize cAMP on demand, employing a red fluorescent cAMP probe (PF2) as a readout tool, and isolating the target pathway through genetic editing (Figure 3). Leveraging spectral separation technology, they achieved simultaneous signal stimulation and measurement. In the experiments, the team mapped the relationship between signal amplitude, noise, and frequency, and derived the information transmission rate. These results not only confirmed the existence of the theoretically predicted optimal frequency but also revealed the presence of effective two-state encoding at this frequency, with the information transmission limit of a single cell approaching 40 bits per hour. All conclusions were based on direct comparisons between closed-form predictions and single-cell data, rather than post-hoc curve fitting.
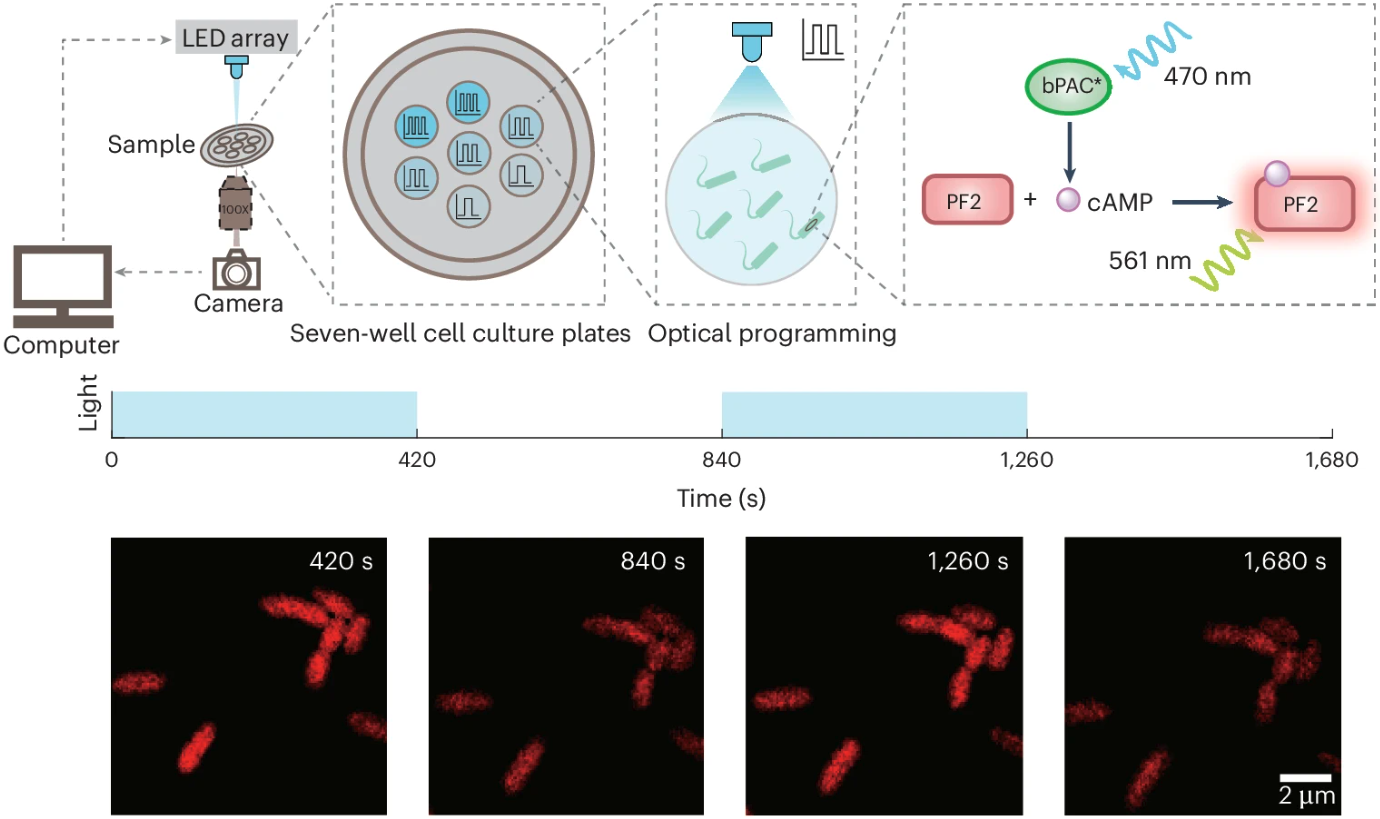
Figure 3 Quantitative analysis of cAMP signalling dynamics under modulated light stimulation at single-cell resolution. Modified from the original paper.
During the research process, the team faced a philosophical consideration regarding the academic positioning of their work: whether the results were "biologically relevant enough" to explain specific phenotypes. Since they could not directly link the optimal frequency to specific virulence programs or adhesion patterns in the short term, the team initially worried that the research was overly abstract when submitting their manuscript. However, feedback from reviewers highlighted a broader value of the study. The theoretical framework applies not only to cAMP but also reveals universal properties of biochemical reactions, in principle, extendable to all second-messenger systems in different bacterial strains and even synthetic cells. This perspective helped the team recognize that abstract theory is not an "escape from reality" but a "powerful lever" for breaking through the constraints of complex biological contexts and guiding research directions and measurement priorities.
In a second study focused on the "Decoding frequency-modulated signals increases information entropy in bacterial second messenger networks," the team adhered to a research rhythm of "theory before experiment." They first derived mathematical equations describing how cells decode frequency-modulated (FM) signals, from which they extracted a concise architecture for a frequency-to-amplitude converter (FAC). This architecture consists of three modules with clearly separated time scales: a wave converter responsible for the synthesis and degradation of cAMP, a thresholding filter activated by Vfr–cAMP, and an integrator for gene expression (Figure 4). This structure was not a theoretical construct but a natural derivation from kinetic laws, enabling precise predictions of how duty cycle and frequency influence steady-state protein levels.
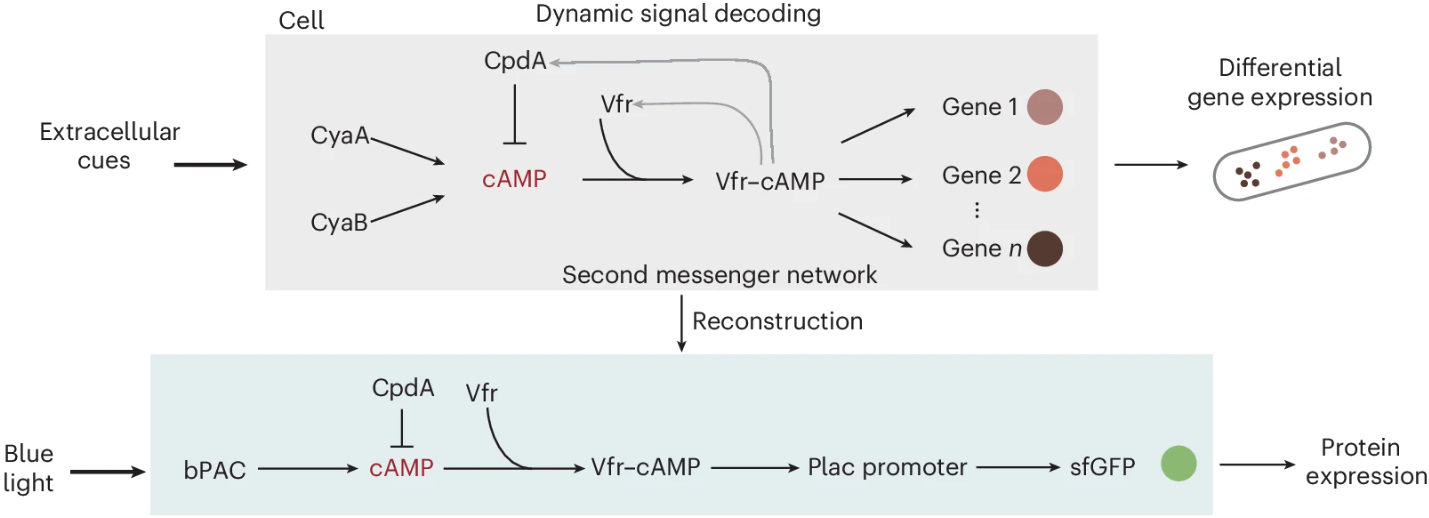
Figure 4 Reconstruction of cAMP second messenger networks and the hierarchical architecture of frequency-to-amplitude signal conversion. Modified from the original paper.
To validate this theoretical framework in living cells, the team reconstructed the cAMP second-messenger pathway in P. aeruginosa. They replaced the native, complex inputs with a bPAC, fixed the expression of the phosphodiesterase CpdA and the effector Vfr using constitutive promoters, and placed a Vfr-responsive promoter upstream of superfolder green fluorescent protein (sfGFP) as a readout element. This setup provided the "control knobs" required by the theory for precise regulation of synthesis (light intensity), degradation (CpdA level), and thresholding (Vfr concentration).
Simultaneously, the team built an automated experimental platform capable of maintaining 96 culture systems in a steady state, programming distinct light signals for each system, sampling hourly, diluting on a scheduled basis, and reading fluorescence signals in situ (Figure 5). While seemingly conventional, this platform provided critical stability, and throughput guarantees for dynamic response research. It maintained the optical density (OD) of the cultures at approximately 0.09 for over 12 hours, effectively avoiding noise interference and ensuring the reproducibility of frequency response profiles. The experimental results were highly consistent with theoretical predictions: by adjusting the effective threshold and duty cycle, the team observed both high-pass and low-pass FAC behaviors, and the analytical model matched the measurement data across multiple orders of magnitude of parameters. This confirmed that the integrator, as theoretically derived, functioned as a "memory element" converting temporal patterns into steady-state protein levels.
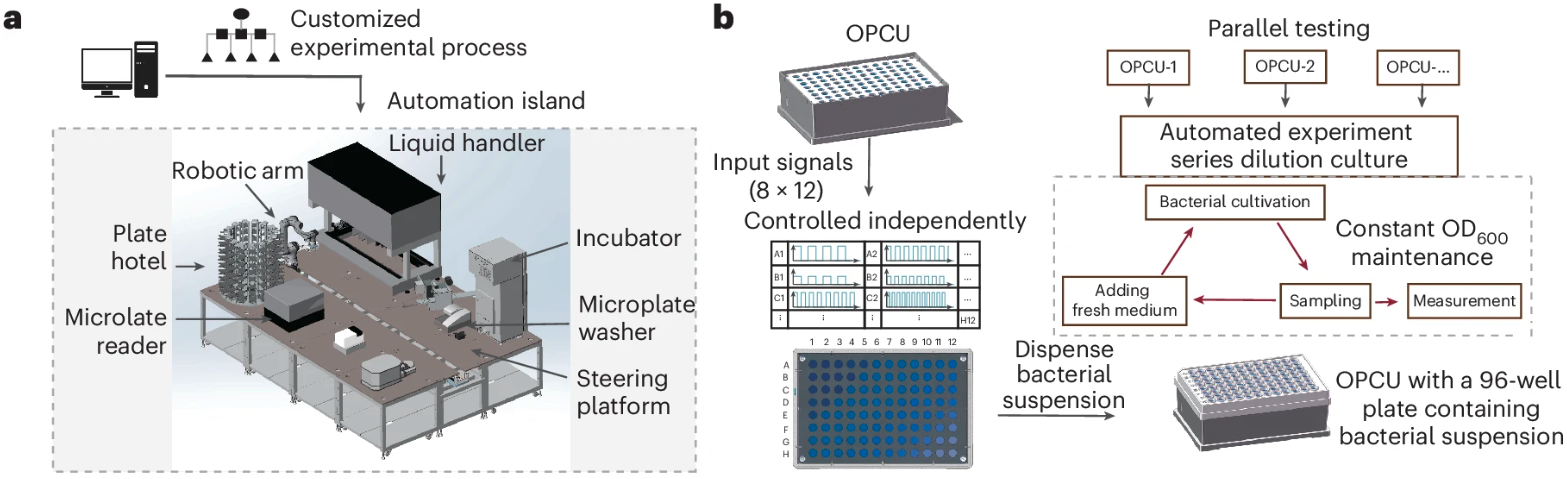
Figure 5 High-throughput automated platform enables the systematic characterization of frequency-decoding cAMP circuit frequency responses. Modified from the original paper.
The significance of this study extends far beyond a single synthetic circuit: FM signals introduce an orthogonal dimension for cellular regulation. In multi-gene systems, when combined with different thresholds, frequency enables the regulation of expression states that cannot be achieved by amplitude alone. In a three-gene demonstration experiment, FM signals increased information capacity by approximately 2 bits, nearly quadrupling the number of distinguishable output states in a realistic noise environment, representing a quantifiable expansion of the cell's accessible state space.
Both studies collectively reinforced the core research workflow of Fan Jin's team: using theoretical derivation to define research boundaries (avoiding an excessively large parameter space), employing simulations to identify potential failure modes, and finally conducting automation-centered experiments to directly validate key theoretical predictions. Fan Jin stated that mathematics does not replace biology but provides clear guidance for biological research. Currently, the team is preparing for an academic conference to be held in Xiamen at the end of October, where they will discuss how to develop a mathematical formalism for living cells and identify the core mathematical tools required.
Fan defined the next frontier as a decoding problem: understanding how cells read frequency-encoded messages. He argues that solving this through a rigorous cycle of analysis, simulation, and experiment will complete a vital scientific circle. "That," he concluded, "is the promise of quantitative synthetic biology."
Original papers:
[1] Xiong, J., Wang, L., Lin, J. et al. Quantifying second-messenger information transmission in bacteria. Nat. Phys. 21, 1009–1018 (2025). https://doi.org/10.1038/s41567-025-02848-2
[2] Zhang, R., Wan, S., Xiong, J. et al. Decoding frequency-modulated signals increases information entropy in bacterial second messenger networks. Nat. Phys. (2025). https://doi.org/10.1038/s41567-025-03030-4

