Autonomous ribosome biogenesis in vitro
Osaka University's Professor Wataru Aoki and his research team have reported a study focusing on the in vitro biogenesis of the Escherichia coli ribosome which marks a significant step forward in advancing our understanding of life's self-replication mechanisms and the development of artificial cells. The original research paper was published in Nature Communications on January 8, 2025.
Ribosome biogenesis is a highly intricate process. The E. coli ribosome, for example, is composed of 57 structural components: three types of ribosomal RNA (rRNA) and 54 types of ribosomal proteins (r-proteins). These components assemble into new ribosomes, aided by nearly 100 accessory proteins that support critical steps such as rRNA processing, post-transcriptional modifications, post-translational modifications of r-proteins, and ribosome folding. Thus, ribosome biogenesis is one of the most complex biological phenomena, requiring the coordinated interplay of about 200 different factors.
Reconstituting such a complex reaction in vitro cannot be achieved through mere trial and error; it requires a guiding principle. To this end, we formulated a simple hypothesis: ribosome biogenesis could be reconstituted in vitro by faithfully mimicking the intracellular environment. To test this hypothesis, we proceeded to construct a reaction system that emulated these conditions.
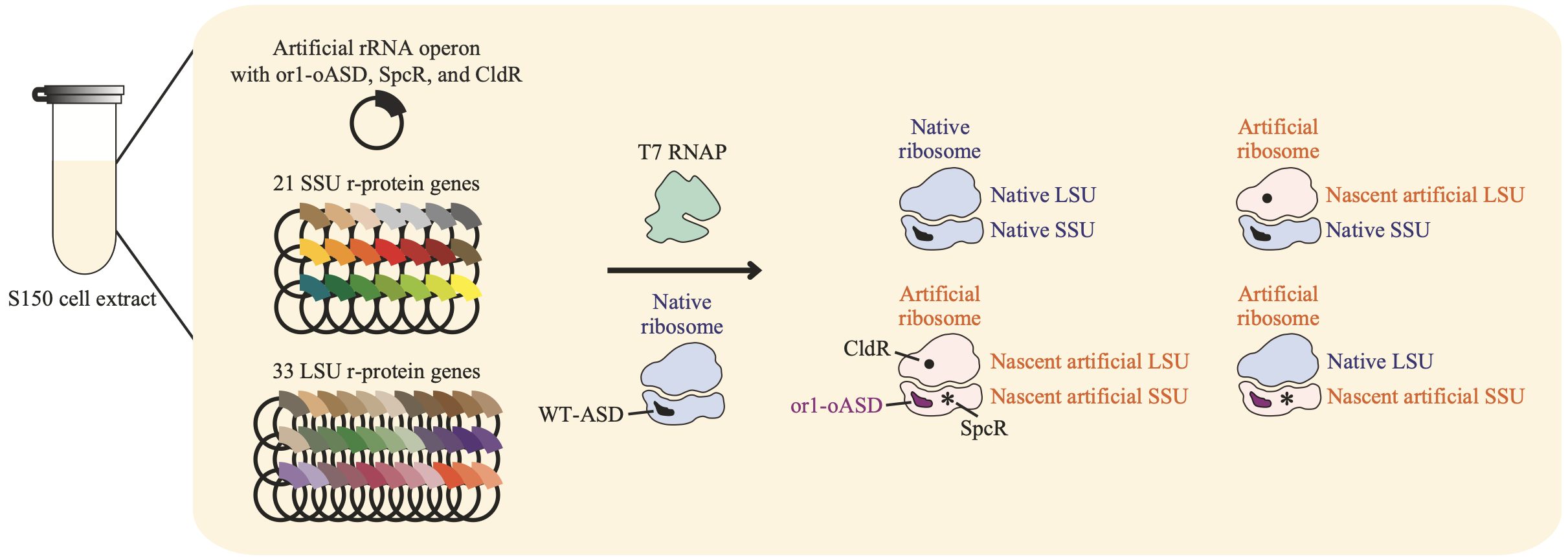
Figure 1 Schematic diagram showing in vitro synthesis of both SSU and LSU in a single reaction space. Modified from the original article.
Specifically, we prepared an E. coli S150 cell extract to serve as the reaction's foundation, as it is thought to contain all 100 accessory proteins required for ribosome biogenesis (Figure 1). The S150 extract is the supernatant fraction obtained after centrifuging an E. coli lysate at 150,000 x g. To better replicate the intracellular chemical environment, we supplemented this extract with various small-molecule compounds abundant in E. coli, such as potassium glutamate, magnesium glutamate, spermidine, and putrescine. Next, we added approximately 50 factors essential for transcription and translation—including NTPs, amino acids, tRNAs, CoA, and NAD—at their proper concentrations. Finally, to initiate the biogenesis process, we introduced native ribosomes, RNA polymerase, the three rRNA genes, and the 54 r-protein genes into the system. Through repeated optimization of these reaction conditions, we achieved the world's first successful in vitro reconstitution of ribosome biogenesis.
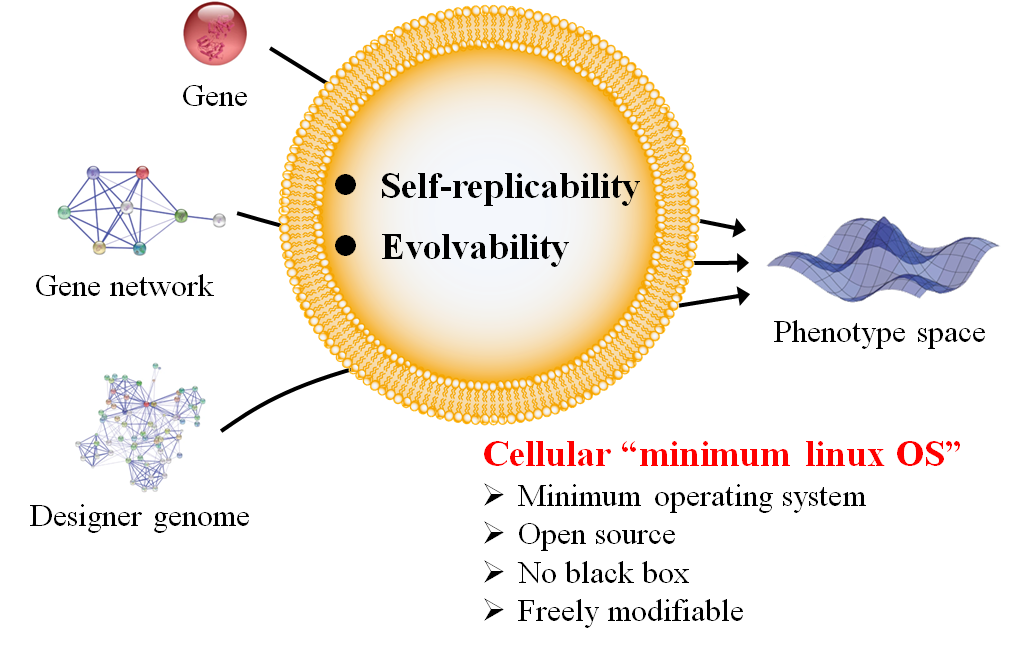
Figure 2 Autonomous replication of artificial cells.
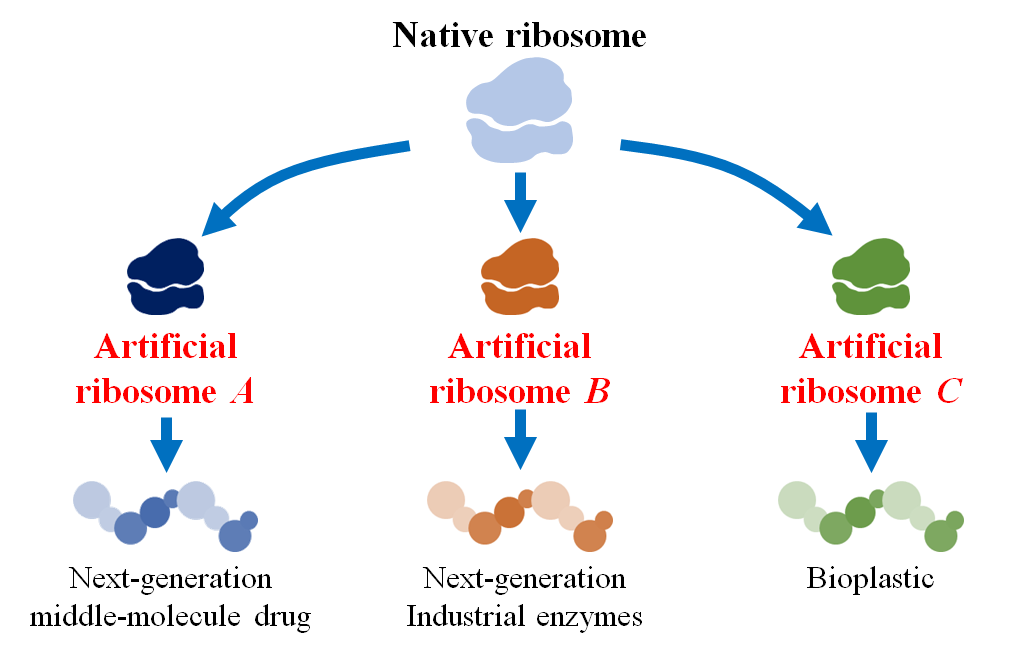
Figure 3 Potential applications of artificial ribosomes.
This achievement is expected to lead to the following two developments. First, it is anticipated to lead to the creation of autonomously replicating artificial cells (Figure 2). The construction of such cells would allow us to engineer them with designer genes, gene networks, and genomes to manifest virtually any phenotype permissible by the laws of physics. Second, this work is expected to greatly expand the diversity of peptides and proteins available for human use (Figure 3). By designing artificial ribosomes that can efficiently polymerize non-canonical monomers with properties different from natural amino acids, it will become possible to create next-generation middle-molecule drugs with superior characteristics, such as oral bioavailability and cell membrane permeability.

Professor Wataru Aoki received his Ph.D. in Agriculture from Kyoto University in 2013. His research career began as a JSPS Research Fellow (DC1) during his doctoral program and continued as a JSPS Postdoctoral Fellow (PD) at Osaka University. In 2015, he was appointed Assistant Professor at the Graduate School of Agriculture, Kyoto University, where he spent eight years managing a laboratory. During this tenure, he led numerous research projects, holding concurrent positions as a JST PRESTO Researcher and a Kyoto University FOREST Principal Investigator. He also contributed to industry-academia collaboration, serving as an Adjunct Researcher at the Kyoto Bio-Analysis Center and an Expert Advisor for Barcodebody Co., Ltd. Since April 2023, he has served as a Professor at the Graduate School of Engineering, Osaka University, where he is dedicated to pioneering research and mentoring the next generation of scientists in the field of biotechnology.
Cite: Kosaka, Y., Miyawaki, Y., Mori, M. et al. Autonomous ribosome biogenesis in vitro. Nat Commun 16, 514 (2025). https://doi.org/10.1038/s41467-025-55853-7

